Retail Food Equivalents for Post-Oral Immunotherapy Dosing in the Omalizumab as Monotherapy and as Adjunct Therapy to Multi-Allergen Oral Immunotherapy in Food-Allergic Children and Adults (OUtMATCH) Clinical Trial
- PMID: 37113037
- PMCID: PMC10147955
- DOI: 10.1016/j.jaip.2022.10.022
Retail Food Equivalents for Post-Oral Immunotherapy Dosing in the Omalizumab as Monotherapy and as Adjunct Therapy to Multi-Allergen Oral Immunotherapy in Food-Allergic Children and Adults (OUtMATCH) Clinical Trial
Abstract
Background: Patients with food allergy may be advised to introduce specific foods into their diets, both to increase tolerance gradually and as next steps after completing oral immunotherapy or other therapeutic interventions. However, the safe use of retail foods depends on the ability to establish the specific allergen protein content of these foods.
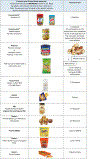
Objective: To develop a systematic approach to estimate the protein content of peanut, milk, egg, wheat, cashew, hazelnut, and walnut in a variety of retail food equivalents for each allergen and associated patient education materials.
Method: We created an algorithm that used a multistep process with information from product food labels, nutrient databases, independent weighing and measuring of foods, and information provided by manufacturers, including certificates of analysis, and e-mail communication to estimate the allergen protein content of multiple retail foods for each of seven allergens. Once a variety of retail food equivalents for each allergen and allergen serving size was determined, we developed participant education handouts, which were reviewed by study teams at 10 food allergy centers, the National Institute of Allergy and Infectious Diseases, and the Consortium for Food Allergy Research coordinating center. After 1 year of use, multiple queries were addressed and the retail food equivalents and educational materials were reviewed and edited.
Results: We identified a variety of retail food equivalents for seven allergens at six serving sizes, and created 48 unique patient education materials.
Conclusion: Our results provide extensive guidance on a variety of retail equivalents for seven foods, and a method to estimate retail food protein equivalents systematically with ongoing reassessment.
Keywords: Allergens; Consortium for Food Allergy Research; Diet; Food allergy; Nutrition; OUtMATCH; Omalizumab; Oral immunotherapy; Retail food equivalents.
Copyright © 2022 American Academy of Allergy, Asthma & Immunology. All rights reserved.
Conflict of interest statement
Conflict of interest:
M.E. Groetch receives royalties from UpToDate, FARE, and Academy of Nutrition and Dietetics; serves on the Medical Advisory Board of IFPIES, as a Senior Advisor to FARE, as a Health Sciences Advisor for APFED and on the editorial board of Journal of Food Allergy; she has no commercial interests to disclose.
K. Mudd has no conflict of interest to report
M. Woch has no conflict of interest to report.
A. Schaible has no conflict of interest to report.
B.E. Gray has no conflict of interest to report and acknowledges her work is supported by Grant Number 1UL1TR002541-01.
J.A. Bird reports grants from NIH-NIAID, Genentech, Aimmune, Astellas, DBV Technologies, Food Allergy Research and Education (FARE), Novartis and Regeneron and personal fees from AllerGenis, Allergy Therapeutics, Before Brands, DBV Technologies, FARE, HAL Allergy, Novartis, and Nutricia.
S.M. Jones reports grants from NIH-NIAID, Food Allergy Research & Education (FARE), Aimmune Therapeutic, DBV Technologies, Astellas, Inc., Sanofi, Inc., Regeneron, Inc., and Genentech, Inc. and personal fees from Food Allergy Research and Education, Aimmune Therapeutics
E.H. Kim reports ad board: ALK, DBV Technologies, Kenota Health, Ukko Inc and consultant to AllerGenis, Allergy Therapeutics Ltd, Belhaven Pharma, Duke Clinical Research Institute, Genentech, Nutricia
B.J. Lanser reports grants and personal fees from Aimmune Therapeutics, DBV Technologies, and Genentech; grants from Regeneron; personal fees from Allergenis, and GlaxoSmithKline; and grant support to his institution from the NIH/NIAID.
J. Poyser co-authorship of this publication does not necessarily constitute endorsement by the NIAID, the NIH or any other agency of the US government.
A.K. Rudman Spergel co-authorship of this publication does not necessarily constitute endorsement by the NIAID, the NIH or any other agency of the US government.
N. Rogers has no conflict of interest to report.
D.C. Babineau has no conflict of interest to report.
W.G.Shreffler reports grants from Aimmune, Angany, DBV, FARE, FASI, Novartis, NIAID, Regeneron, personal fees from Aimmune, DBV, Merk, Novartis, Regeneron and UpToDate
S.H. Sicherer reports royalty payments from UpToDate and from Johns Hopkins University Press; grants to his institution from the National Institute of Allergy and Infectious Diseases, and from Food Allergy Research and Education; and personal fees from the American Academy of Allergy, Asthma and Immunology as Deputy Editor of the Journal of Allergy and Clinical Immunology: In Practice, outside of the submitted work.
J.M.Spergel reports grant support from Novartis, Regeneron-Sanofi, National Institute of Allergy and Infectious Diseases and Food Allergy Research Education; royalties from Uptodate; and consultant agreements from Regeneron, Sanofi, and Novartis.
B.P.Vickery reports grants from Abbott, grants and personal fees from Aimmune, grants from Alladapt, personal fees from AllerGenis, personal fees from Aravax, grants and personal fees from DBV, grants and personal fees from FARE, grants from Genentech, personal fees from Moonlight Therapeutics, grants from NIH-NIAID, personal fees from Reacta Biosciences, grants and personal fees from Regeneron, grants from Siolta.
R.S. Chinthrajah reports grants from NIAID, CoFAR, Aimmune Therapeutics, DBV Technologies, Astellas, Regeneron, FARE, Stanford Maternal and Child Health Research Institute (MCHRI); is an advisory board member for Alladapt Therapeutics, Novartis, Genentech, Sanofi, Allergenis, Intrommune Therapeutics, and Nutricia, outside the submitted work.
R.A. Wood receives research support from NIAID, Aimmune, Astellas, DBV, FARE, Genentech, Novartis, Regeneron, and Siolta, and royalties from Up To Date
Figures



References
-
- Meyer R, Wright K, Vieira MC, Chong KW, Chatchatee P, Vlieg-Boerstra BJ, et al. International survey on growth indices and impacting factors in children with food allergies. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association. 2019;32(2):175–84. - PubMed
-
- Dyer AA, Negris OR, Gupta RS, Bilaver LA. Food allergy: how expensive are they? Current opinion in allergy and clinical immunology. 2020;20(2):188–93. - PubMed
-
- Clark S, Espinola J, Rudders SA, Banerji A, Camargo CA Jr Frequency of US emergency department visits for food-related acute allergic reactions. The Journal of allergy and clinical immunology. 2011;127(3):682–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR002541/TR/NCATS NIH HHS/United States
- UL1 TR003107/TR/NCATS NIH HHS/United States
- UM1 AI130780/AI/NIAID NIH HHS/United States
- UL1 TR003098/TR/NCATS NIH HHS/United States
- UM2 AI130836/AI/NIAID NIH HHS/United States
- UM1 AI130934/AI/NIAID NIH HHS/United States
- UL1 TR002535/TR/NCATS NIH HHS/United States
- UM2 AI117870/AI/NIAID NIH HHS/United States
- UM1 AI130936/AI/NIAID NIH HHS/United States
- UM1 AI130781/AI/NIAID NIH HHS/United States
- UL1 TR002489/TR/NCATS NIH HHS/United States
- UL1 TR000170/TR/NCATS NIH HHS/United States
- UL1 TR001102/TR/NCATS NIH HHS/United States
- UM1 AI130570/AI/NIAID NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
- UM1 AI130838/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

