Pathogenesis of cerebral amyloid angiopathy caused by chaotic glymphatics-Mini-review
- PMID: 37113157
- PMCID: PMC10126375
- DOI: 10.3389/fnins.2023.1180237
Pathogenesis of cerebral amyloid angiopathy caused by chaotic glymphatics-Mini-review
Abstract
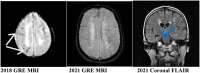
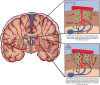
Cerebral amyloid angiopathy (CAA) is a common cause of lobar intracerebral hemorrhage in the elderly. It is also associated pathologically with Alzheimer's disease (AD). Both CAA and AD share similar pathology of deposition amyloid beta fibrils (Aβ). Aβ is deposited mainly in the neurites in AD and vascular walls in CAA. Aβ is formed inside the brain parenchyma from the amyloid precursor protein. It is easier to understand how Aβ is deposited in the cerebral neurites in AD. However, the pathogenesis of CAA is still largely unknown. It is difficult to understand or visualize how Aβ fibrils formed inside the brain can be deposited against the cerebral perfusion pressure to be deposited in the cerebral and meningeal arterial walls. We encountered an unusual clinical case of acute aneurysmal subarachnoid hemorrhage which was followed after a few years with localized CAA involving mainly the sites of the subarachnoid hemorrhage. We reviewed the formation of Aβ and postulated how the Aβ fibrils are transported retrogradely toward the cerebral arteries and deposited in the arterial walls resulting in the final pathology of CAA. There is a clear disturbance of the glymphatic system, the aquaporin-4 channel, and the parenchymal border macrophages.
Keywords: Aβ clearance; aquaporin-4; astrocytes; cerebral amyloid angiopathy; glymphatics; parenchymal border macrophages.
Copyright © 2023 Lui, Alcaide, Knowlton, Ysit and Zhong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Charidimou A., Boulouis G., Frosch M. P., Baron J. C., Pasi M., Albucher J. F., et al. (2022). The Boston criteria version 2.0 for cerebral amyloid angiopathy: A multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol. 21, 714–725. 10.1016/S1474-4422(22)00208-3 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

