Steady electrocorticogram characteristics predict specific stress-induced behavioral phenotypes
- PMID: 37113159
- PMCID: PMC10126346
- DOI: 10.3389/fnins.2023.1047848
Steady electrocorticogram characteristics predict specific stress-induced behavioral phenotypes
Abstract
Introduction: Depending on the individual, exposure to an intense stressor may, or may not, lead to a stress-induced pathology. Predicting the physiopathological evolution in an individual is therefore an important challenge, at least for prevention. In this context, we developed an ethological model of simulated predator exposure in rats: we call this the multisensorial stress model (MSS). We hypothesized that: (i) MSS exposure can induce stress-induced phenotypes, and (ii) an electrocorticogram (ECoG) recorded before stress exposure can predict phenotypes observed after stress.
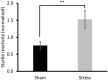
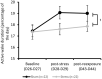
Methods: Forty-five Sprague Dawley rats were equipped with ECoG telemetry and divided into two groups. The Stress group (n = 23) was exposed to an MSS that combined synthetic fox feces odor deposited on filter paper, synthetic blood odor, and 22 kHz rodent distress calls; the Sham group (n = 22) was not exposed to any sensorial stimulus. Fifteen days after initial exposure, the two groups were re-exposed to a context that included a filter paper soaked with water as a traumatic object (TO) reminder. During this re-exposure, freezing behavior and avoidance of the filter paper were measured.
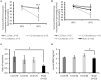
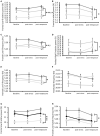
Results: Three behaviors were observed in the Stress group: 39% developed a fear memory phenotype (freezing, avoidance, and hyperreactivity); 26% developed avoidance and anhedonia; and 35% made a full recovery. We also identified pre-stress ECoG biomarkers that accurately predicted cluster membership. Decreased chronic 24 h frontal Low θ relative power was associated with resilience; increased frontal Low θ relative power was associated with fear memory; and decreased parietal β2 frequency was associated with the avoidant-anhedonic phenotype.
Discussion: These predictive biomarkers open the way to preventive medicine for stress-induced diseases.
Keywords: EEG; fear; multisensorial stress; theta; vulnerability; β2.
Copyright © 2023 Desnouveaux, Poly, Edmond, Aphezberro, Coulon, Boutet, Le Coz, Fargeau, Linard, Caillol, Duffaud, Servonnet, Ferhani, Trousselard, Taudon, Canini and Claverie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

