EuMAR: a roadmap towards a prospective, cycle-by-cycle registry of medically assisted reproduction in Europe
- PMID: 37113274
- PMCID: PMC10126319
- DOI: 10.1093/hropen/hoad011
EuMAR: a roadmap towards a prospective, cycle-by-cycle registry of medically assisted reproduction in Europe
Abstract
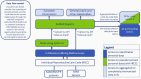
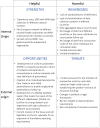
More than 20 years ago, the survey of activities in medically assisted reproduction (MAR) was initiated in Europe and resulted in cross-sectional annual reports, as issued by the European IVF Monitoring (EIM) consortium of ESHRE. Over time, these reports mirror the continuous development of the technologies and contribute to increased transparency and surveillance of reproductive care. Meanwhile, progressive changes of existing treatment modalities and the introduction of new technologies resulted in the need of a cumulative approach in the assessment of treatment outcomes, which warrants a prospective cycle-by-cycle data registry on MAR activities, including fertility preservation. This change in the paradigm of data collection in Europe towards the construction of cumulative outcome results is expected to generate additional insights into cross-institutional but also cross-border movements of patients and reproductive material. This is essential to improve vigilance and surveillance. The European monitoring of Medically Assisted Reproduction (EuMAR) project, co-funded by the European Union, will establish a registry for the transnational collection of prospective cycle-by-cycle MAR and fertility preservation data on the basis of an individual reproductive care code (IRCC). The rationale for the project and the objectives are presented here.
Keywords: ART; Europe; epidemiology; fertility preservation; medically assisted reproduction; registry; surveillance; vigilance.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
J.S. reports speaker’s fees from Ferring and Merck, support to attend conferences from Ferring, Merck and Good Life, and participation in an advisory board for Merck, outside the scope of the current work. The other authors have no conflicts of interest to declare.
Figures

References
-
- Andersen AN, Gianaroli L, Felberbaum R, de Mouzon J, Nygren KG; European IVF-monitoring programme (EIM), European Society of Human Reproduction and Embryology (ESHRE). Assisted reproductive technology in Europe, 2001. Results generated from European registers by ESHRE. Hum Reprod 2005;20:1158–1176. - PubMed
-
- De Geyter C. Assisted reproductive technology: impact on society and need for surveillance. Best Pract Res Clin Endocrinol Metab 2019;33:3–8. - PubMed
-
- De Geyter C, Wyns C, Mocanu E, de Mouzon J, Calhaz-Jorge C.. Data collection systems in ART must follow the pace of change in clinical practice. Hum Reprod 2016;31:2160–2163. - PubMed
-
- Ferraretti AP, Nygren K, Andersen AN, de Mouzon J, Kupka M, Calhaz-Jorge C, Wyns C, Gianaroli L, Goossens V. ; European IVF-Monitoring Consortium (EIM), for the European Society of Human Reproduction and Embryology (ESHRE). Trends over 15 years in ART in Europe: an analysis of 6 million cycles. Hum Reprod Open 2017;2017:hox012. - PMC - PubMed
LinkOut - more resources
Full Text Sources
