Stakeholders' Perspective on the Integration of Pharmacovigilance Activities into the Eritrean Healthcare System: A Qualitative Study
- PMID: 37113312
- PMCID: PMC10128073
- DOI: 10.2147/RMHP.S398811
Stakeholders' Perspective on the Integration of Pharmacovigilance Activities into the Eritrean Healthcare System: A Qualitative Study
Abstract
Purpose: Understanding the integration of pharmacovigilance activities in the healthcare system and identifying existing hindering factors systematically through the eyes of stakeholders has paramount importance to realize a successful integration. Thus, this study aimed to assess the perspectives of the stakeholders of the Eritrean Pharmacovigilance Center (EPC) on the integration of pharmacovigilance activities into the Eritrean healthcare system.
Methods: An exploratory qualitative assessment of the integration of pharmacovigilance activities into the healthcare system was conducted. Key informant interviews were conducted among the major stakeholders of the EPC via face-to-face and telephone interviews. Data were collected between October 2020 and February 2021, and analyzed through thematic framework analysis.
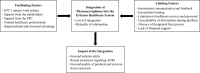
Results: A total of 11 interviews were completed. The integration of the EPC in the healthcare system was rated as good and encouraging except in the National Blood Bank, and Health Promotion. The relationship between the EPC and Public health programs was described as mutual with an eminent impact. Several facilitating factors for integration were identified namely: the unique work culture of the EPC; provision of basic and advanced training; motivating and recognizing healthcare professionals in vigilance activities; and the financial and technical support provided to the EPC from national and international stakeholders. On the other hand, the absence of concrete communication systems, inconsistencies in training and communication, the absence of data-sharing mechanisms and policies, and the absence of designated pharmacovigilance focal persons were identified as barriers to successful integration.
Conclusion: Integration of the EPC in the healthcare system was found to be commendable except in some areas of the healthcare system. Therefore, the EPC should work towards identifying further areas of integration, mitigate the identified limitations and at the same time sustain the integration already initiated.
Keywords: facilitating factors; key informant interview; limiting factors; thematic framework analysis.
© 2023 Abraham et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Effective integration of pharmacovigilance systems at public health facilities in resource-limited settings: A qualitative study.Res Social Adm Pharm. 2020 Aug;16(8):1111-1116. doi: 10.1016/j.sapharm.2019.11.010. Epub 2019 Nov 18. Res Social Adm Pharm. 2020. PMID: 31812500
-
A Qualitative Study of Stakeholders' Views on Pharmacovigilance System, Policy, and Coordination in Pakistan.Front Pharmacol. 2022 Jun 9;13:891954. doi: 10.3389/fphar.2022.891954. eCollection 2022. Front Pharmacol. 2022. PMID: 35754475 Free PMC article.
-
Stakeholders' knowledge, attitudes and practices to pharmacovigilance and adverse drug reaction reporting in clinical trials: a mixed methods study.Eur J Clin Pharmacol. 2020 Oct;76(10):1363-1372. doi: 10.1007/s00228-020-02921-0. Epub 2020 Jun 7. Eur J Clin Pharmacol. 2020. PMID: 32507924
-
Healthcare stakeholders' perceptions and experiences of factors affecting the implementation of critical care telemedicine (CCT): qualitative evidence synthesis.Cochrane Database Syst Rev. 2021 Feb 18;2(2):CD012876. doi: 10.1002/14651858.CD012876.pub2. Cochrane Database Syst Rev. 2021. PMID: 33599282 Free PMC article.
-
Measuring integrated care.Dan Med Bull. 2011 Feb;58(2):B4245. Dan Med Bull. 2011. PMID: 21299927 Review.
References
-
- Swain S, Patra CN. Impact of pharmacovigilance in healthcare system: regulatory perspective. Pharmaceut Reg Affairs. 2014;3:5. doi:10.4172/2167-7689.1000e143 - DOI
LinkOut - more resources
Full Text Sources