An Overview of the Use of Precision Population Medicine in Cancer Care: First of a Series
- PMID: 37113463
- PMCID: PMC10129036
- DOI: 10.7759/cureus.37889
An Overview of the Use of Precision Population Medicine in Cancer Care: First of a Series
Abstract
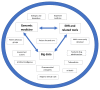
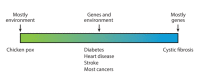
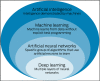
Advances in science and technology in the past century and a half have helped improve disease management, prevention, and early diagnosis and better health maintenance. These have led to a longer life expectancy in most developed and middle-income countries. However, resource- and infrastructure-scarce countries and populations have not enjoyed these benefits. Furthermore, in every society, including in developed nations, the lag time from new advances, either in the laboratory or from clinical trials, to using those findings in day-to-day medical practice often takes many years and sometimes close to or longer than a decade. A similar trend is seen in the application of "precision medicine" (PM) in terms of improving population health (PH). One of the reasons for such lack of application of precision medicine in population health is the misunderstanding of equating precision medicine with genomic medicine (GM) as if they are the same. Precision medicine needs to be recognized as encompassing genomic medicine in addition to other new developments such as big data analytics, electronic health records (EHR), telemedicine, and information communication technology. By leveraging these new developments together and applying well-tested epidemiological concepts, it can be posited that population/public health can be improved. In this paper, we take cancer as an example of the benefits of recognizing the potential of precision medicine in applying it to population/public health. Breast cancer and cervical cancer are taken as examples to demonstrate these hypotheses. There exists significant evidence already to show the importance of recognizing "precision population medicine" (PPM) in improving cancer outcomes not only in individual patients but also for its applications in early detection and cancer screening (especially in high-risk populations) and achieving those goals in a more cost-efficient manner that can reach resource- and infrastructure-scarce societies and populations. This is the first report of a series that will focus on individual cancer sites in the future.
Keywords: artificial intelligence; big data; genomic medicine; multi-cancer early detection; precision medicine; precision population medicine.
Copyright © 2023, Yang et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







Similar articles
-
Precision Population Cancer Medicine in Cancer of the Uterine Cervix: A Potential Roadmap to Eradicate Cervical Cancer.Cureus. 2024 Feb 6;16(2):e53733. doi: 10.7759/cureus.53733. eCollection 2024 Feb. Cureus. 2024. PMID: 38455773 Free PMC article. Review.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
THE FUTURE OF MEDICINE, healthcare innovation through precision medicine: policy case study of Qatar.Life Sci Soc Policy. 2020 Nov 1;16(1):12. doi: 10.1186/s40504-020-00107-1. Life Sci Soc Policy. 2020. PMID: 33129349 Free PMC article. Review.
-
Nanotechnology: an evidence-based analysis.Ont Health Technol Assess Ser. 2006;6(19):1-43. Epub 2006 Nov 1. Ont Health Technol Assess Ser. 2006. PMID: 23074489 Free PMC article.
-
The effectiveness of web-based programs on the reduction of childhood obesity in school-aged children: A systematic review.JBI Libr Syst Rev. 2012;10(42 Suppl):1-14. doi: 10.11124/jbisrir-2012-248. JBI Libr Syst Rev. 2012. PMID: 27820152
Cited by
-
Precision Population Cancer Medicine in Brain Tumors: A Potential Roadmap to Improve Outcomes and Strategize the Steps to Bring Interdisciplinary Interventions.Cureus. 2024 Oct 12;16(10):e71305. doi: 10.7759/cureus.71305. eCollection 2024 Oct. Cureus. 2024. PMID: 39529768 Free PMC article. Review.
-
An Interdisciplinary Perspective on Improving Cancer Care in the State of Mississippi as an Example of Cancer Care Improvements in the Global South.Cureus. 2025 Jan 3;17(1):e76865. doi: 10.7759/cureus.76865. eCollection 2025 Jan. Cureus. 2025. PMID: 39758867 Free PMC article. Review.
-
Usage of the National Cancer Institute Cancer Research Data Commons by Researchers: A Scoping Review of the Literature.JCO Clin Cancer Inform. 2024 Nov;8:e2400116. doi: 10.1200/CCI.24.00116. Epub 2024 Nov 13. JCO Clin Cancer Inform. 2024. PMID: 39536277 Free PMC article.
-
India's Potential as a Leader in Cancer Care Progress in the Future: A Synthetic Interdisciplinary Perspective.Cureus. 2024 Oct 5;16(10):e70892. doi: 10.7759/cureus.70892. eCollection 2024 Oct. Cureus. 2024. PMID: 39376975 Free PMC article. Review.
-
Racial Differences in Radium-223 Treatment Response and Adverse Effects in a Small-Cohort, Pilot, Hypothesis-Generating Observational Study.Ochsner J. 2025 Summer;25(2):75-76. doi: 10.31486/toj.25.0023. Ochsner J. 2025. PMID: 40538615 Free PMC article. No abstract available.
References
-
- Individual, health system, and contextual barriers and facilitators for the implementation of clinical practice guidelines: a systematic metareview. Correa VC, Lugo-Agudelo LH, Aguirre-Acevedo DC, Contreras JA, Borrero AM, Patiño-Lugo DF, Valencia DA. Health Res Policy Syst. 2020;18:74. - PMC - PubMed
-
- Machine learning for precision medicine. MacEachern SJ, Forkert ND. Genome. 2021;64:416–425. - PubMed
-
- A short history of transfusion medicine. Greenwalt TJ. Transfusion. 1997;37:550–563. - PubMed
-
- Precision medicine in cancer prevention: exploring applications in Mississippi and beyond. Vijayakumar S, Roberts PR, Packianathan S. https://issuu.com/jmsmamanagingeditor/docs/jan_2017_final/6. J Miss State Med Assoc. 2017;58:4–7. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
