Basal NAD(H) redox state permits hydrogen peroxide-induced mesenteric artery dilatation
- PMID: 37114864
- PMCID: PMC11714382
- DOI: 10.1113/JP284195
Basal NAD(H) redox state permits hydrogen peroxide-induced mesenteric artery dilatation
Abstract
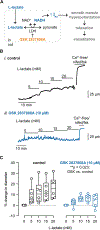
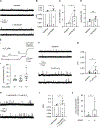
Smooth muscle voltage-gated K+ (Kv) channels in resistance arteries control vascular tone and contribute to the coupling of blood flow with local metabolic activity. Members of the Kv1 family are expressed in vascular smooth muscle and are modulated upon physiological elevation of local metabolites, including the glycolytic end-product l-lactate and superoxide-derived hydrogen peroxide (H2 O2 ). Here, we show that l-lactate elicits vasodilatation of small-diameter mesenteric arteries in a mechanism that requires lactate dehydrogenase (LDH). Using the inside-out configuration of the patch clamp technique, we show that increases in NADH that reflect LDH-mediated conversion of l-lactate to pyruvate directly stimulate the activity of single Kv1 channels and significantly enhance the sensitivity of Kv1 activity to H2 O2 . Consistent with these findings, H2 O2 -evoked vasodilatation was significantly greater in the presence of 10 mM l-lactate relative to lactate-free conditions, yet was abolished in the presence of 10 mM pyruvate, which shifts the LDH reaction towards the generation of NAD+ . Moreover, the enhancement of H2 O2 -induced vasodilatation was abolished in arteries from double transgenic mice with selective overexpression of the intracellular Kvβ1.1 subunit in smooth muscle cells. Together, our results indicate that the Kvβ complex of native vascular Kv1 channels serves as a nodal effector for multiple redox signals to precisely control channel activity and vascular tone in the face of dynamic tissue-derived metabolic cues. KEY POINTS: Vasodilatation of mesenteric arteries by elevated external l-lactate requires its conversion by lactate dehydrogenase. Application of either NADH or H2 O2 potentiates single Kv channel currents in excised membrane patches from mesenteric artery smooth muscle cells. The binding of NADH enhances the stimulatory effects of H2 O2 on single Kv channel activity. The vasodilatory response to H2 O2 is differentially modified upon elevation of external l-lactate or pyruvate. The presence of l-lactate enhances the vasodilatory response to H2 O2 via the Kvβ subunit complex in smooth muscle.
Keywords: calcium signalling; endothelium; metabolism; smooth muscle; voltage-gated potassium channels.
© 2023 The Authors. The Journal of Physiology © 2023 The Physiological Society.
Conflict of interest statement
Figures


References
-
- Ahmed K, Tunaru S, Tang C, Muller M, Gille A, Sassmann A, Hanson J & Offermanns S. (2010). An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab 11, 311–319. - PubMed
-
- Barlow RS & White RE. (1998). Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. The American journal of physiology 275, H1283–1289. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

