Long-term safety and efficacy of selumetinib in children with neurofibromatosis type 1 on a phase 1/2 trial for inoperable plexiform neurofibromas
- PMID: 37115514
- PMCID: PMC10547508
- DOI: 10.1093/neuonc/noad086
Long-term safety and efficacy of selumetinib in children with neurofibromatosis type 1 on a phase 1/2 trial for inoperable plexiform neurofibromas
Abstract
Background: Selumetinib shrank inoperable symptomatic plexiform neurofibromas (PN) in children with neurofibromatosis type 1 (NF1) and provided clinical benefit for many in our previously published phase 1/2 clinical trials (SPRINT, NCT01362803). At the data cutoff (DCO) of the prior publications, 65% of participants were still receiving treatment. This report presents up to 5 years of additional safety and efficacy data from these studies.
Methods: This manuscript includes data from the phase 1 and phase 2, stratum 1 study which included participants with clinically significant PN-related morbidity. Participants received continuous selumetinib dosing (1 cycle = 28 days). Safety and efficacy data through February 27, 2021 are included. PN response assessed by volumetric magnetic resonance imaging analysis: Confirmed partial response (cPR) ≥20% decrease from baseline on 2 consecutive evaluations. Phase 2 participants completed patient-reported outcome measures assessing tumor pain intensity (Numeric Rating Scale-11) and interference of pain in daily life (pain interference index).
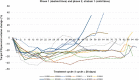
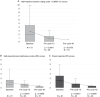
Results: For the 74 children (median age 10.3 years; range 3-18.5) enrolled, overall cPR rate was 70% (52/74); median duration of treatment was 57.5 cycles (range 1-100). Responses were generally sustained with 59% (44) lasting ≥ 12 cycles. Tumor pain intensity (n = 19, P = .015) and pain interference (n = 18, P = .0059) showed durable improvement from baseline to 48 cycles. No new safety signals were identified; however, some developed known selumetinib-related adverse events (AEs) for the first time after several years of treatment.
Conclusions: With up to 5 years of additional selumetinib treatment, most children with NF1-related PN had durable tumor shrinkage and sustained improvement in pain beyond that previously reported at 1 year. No new safety signals were identified; however, ongoing monitoring for known selumetinib-related AEs is needed while treatment continues.
Keywords: MEK Inhibitors; Neurofibromatosis type 1; Plexiform Neurofibromas.
Published by Oxford University Press on behalf of the Society for Neuro-Oncology 2023.
Conflict of interest statement
Dr. Michael J Fisher and Dr. Jaishri O Blakeley have been paid advisors for Springworks Therapeutics. The authors report no other financial conflicts of interest. Dr. Miriam Bornhorst has been a paid advisor for Alexion, AstraZeneca Rare Disease.
Figures



Similar articles
-
Selumetinib in Children with Inoperable Plexiform Neurofibromas.N Engl J Med. 2020 Apr 9;382(15):1430-1442. doi: 10.1056/NEJMoa1912735. Epub 2020 Mar 18. N Engl J Med. 2020. PMID: 32187457 Free PMC article. Clinical Trial.
-
Selumetinib in children with neurofibromatosis type 1 and asymptomatic inoperable plexiform neurofibroma at risk for developing tumor-related morbidity.Neuro Oncol. 2022 Nov 2;24(11):1978-1988. doi: 10.1093/neuonc/noac109. Neuro Oncol. 2022. PMID: 35467749 Free PMC article. Clinical Trial.
-
Activity of Selumetinib in Neurofibromatosis Type 1-Related Plexiform Neurofibromas.N Engl J Med. 2016 Dec 29;375(26):2550-2560. doi: 10.1056/NEJMoa1605943. N Engl J Med. 2016. PMID: 28029918 Free PMC article. Clinical Trial.
-
Selumetinib for children with neurofibromatosis type 1 and plexiform neurofibromas that can't be removed by surgery, and impact on how the condition affects caregivers: a plain language summary.J Comp Eff Res. 2025 Mar;14(3):e240184. doi: 10.57264/cer-2024-0184. Epub 2025 Jan 21. J Comp Eff Res. 2025. PMID: 39835668 Free PMC article. Review.
-
A Review of Selumetinib in the Treatment of Neurofibromatosis Type 1-Related Plexiform Neurofibromas.Ann Pharmacother. 2022 Jun;56(6):716-726. doi: 10.1177/10600280211046298. Epub 2021 Sep 18. Ann Pharmacother. 2022. PMID: 34541874 Review.
Cited by
-
Serum creatine kinase elevation following tyrosine kinase inhibitor treatment in cancer patients: Symptoms, mechanism, and clinical management.Clin Transl Sci. 2024 Nov;17(11):e70053. doi: 10.1111/cts.70053. Clin Transl Sci. 2024. PMID: 39473122 Free PMC article. Review.
-
A Mixed Methods Study of Medication Adherence in Adults with Neurofibromatosis Type 1 (NF1) on a Clinical Trial of Selumetinib.Cancers (Basel). 2025 Jan 17;17(2):295. doi: 10.3390/cancers17020295. Cancers (Basel). 2025. PMID: 39858077 Free PMC article.
-
MEK-SHP2 inhibition prevents tibial pseudarthrosis caused by NF1 loss in Schwann cells and skeletal stem/progenitor cells.Sci Transl Med. 2024 Jun 26;16(753):eadj1597. doi: 10.1126/scitranslmed.adj1597. Epub 2024 Jun 26. Sci Transl Med. 2024. PMID: 38924432 Free PMC article.
-
The Combination of HSP90 Inhibitors and Selumetinib Reinforces the Inhibitory Effects on Plexiform Neurofibromas.Cancers (Basel). 2025 Jul 16;17(14):2359. doi: 10.3390/cancers17142359. Cancers (Basel). 2025. PMID: 40723243 Free PMC article.
-
Expanding a precision medicine platform for malignant peripheral nerve sheath tumors: New patient-derived orthotopic xenografts, cell lines and tumor entities.Mol Oncol. 2024 Apr;18(4):895-917. doi: 10.1002/1878-0261.13534. Epub 2023 Oct 20. Mol Oncol. 2024. PMID: 37798904 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

