Fetal and neonatal dioxin exposure causes sex-specific metabolic alterations in mice
- PMID: 37115651
- PMCID: PMC10306400
- DOI: 10.1093/toxsci/kfad042
Fetal and neonatal dioxin exposure causes sex-specific metabolic alterations in mice
Abstract
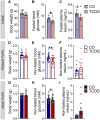
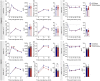
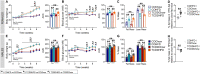
Epidemiological studies report associations between early-life exposure to persistent organic pollutants (POPs) and impaired metabolic homeostasis in adulthood. We investigated the impact of early-life exposure to low-dose 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD or 'dioxin') on the establishment of β-cell area during the perinatal period, as well as β-cell health and glucose homeostasis later in life. Adult female mice were injected with either corn oil (CO; vehicle control) or TCDD (20 ng/kg/day) 2×/week throughout mating, pregnancy, and lactation; offspring were thus indirectly exposed to maternal TCDD in utero and during lactation, with pollutant exposure ending at weaning. All offspring were maintained on chow diet from weaning until 12-17 weeks of age, after which a subset of CO- and TCDD-exposed offspring were transferred to a 45% high fat diet (HFD) as a metabolic stressor for an additional 10 weeks. TCDD significantly upregulated cytochrome P450 1a1 (Cyp1a1) gene expression in offspring pancreas at birth and weaning, indicating that maternal TCDD directly reaches the developing pancreas. TCDD-exposed pups were transiently hypoglycemic at birth and females were born with reduced % β-cell area, which persisted into adulthood. Early-life TCDD exposure had no persistent long-term effects on glucose homeostasis in chow-fed offspring, but when transferred to HFD, TCDD-exposed female offspring had a delayed onset of HFD-induced hyperglycemia, more pronounced HFD-induced hyperinsulinemia, and increase % PCNA+ β-cells compared with CO-exposed female offspring. This study demonstrates that early-life exposure of mice to TCDD has modest effects on metabolic health in chow-fed offspring but alters metabolic adaptability to HFD feeding in females.
Keywords: diabetes; dioxin; early-life pollutant exposure; hypoglycemia; metabolic adaptability; β-cell mass.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society of Toxicology.
Figures





References
-
- Aerts R., Van Overmeire I., Colles A., Andjelković M., Malarvannan G., Poma G., Den Hond E., Van de Mieroop E., Dewolf M.-C., Charlet F., et al. (2019). Determinants of persistent organic pollutant (POP) concentrations in human breast milk of a cross-sectional sample of primiparous mothers in Belgium. Environ. Int. 131, 104979. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

