Pressure overload induces ISG15 to facilitate adverse ventricular remodeling and promote heart failure
- PMID: 37115698
- PMCID: PMC10145941
- DOI: 10.1172/JCI161453
Pressure overload induces ISG15 to facilitate adverse ventricular remodeling and promote heart failure
Abstract
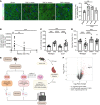
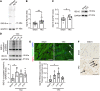
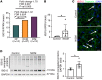
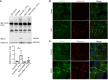
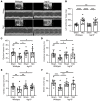
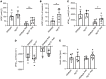
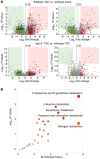
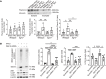
Inflammation promotes adverse ventricular remodeling, a common antecedent of heart failure. Here, we set out to determine how inflammatory cells affect cardiomyocytes in the remodeling heart. Pathogenic cardiac macrophages induced an IFN response in cardiomyocytes, characterized by upregulation of the ubiquitin-like protein IFN-stimulated gene 15 (ISG15), which posttranslationally modifies its targets through a process termed ISGylation. Cardiac ISG15 is controlled by type I IFN signaling, and ISG15 or ISGylation is upregulated in mice with transverse aortic constriction or infused with angiotensin II; rats with uninephrectomy and DOCA-salt, or pulmonary artery banding; cardiomyocytes exposed to IFNs or CD4+ T cell-conditioned medium; and ventricular tissue of humans with nonischemic cardiomyopathy. By nanoscale liquid chromatography-tandem mass spectrometry, we identified the myofibrillar protein filamin-C as an ISGylation target. ISG15 deficiency preserved cardiac function in mice with transverse aortic constriction and led to improved recovery of mouse hearts ex vivo. Metabolomics revealed that ISG15 regulates cardiac amino acid metabolism, whereas ISG15 deficiency prevented misfolded filamin-C accumulation and induced cardiomyocyte autophagy. In sum, ISG15 upregulation is a feature of pathological ventricular remodeling, and protein ISGylation is an inflammation-induced posttranslational modification that may contribute to heart failure development by altering cardiomyocyte protein turnover.
Keywords: Cardiology; Heart failure.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

