Sex-specific effects of CD248 on metabolism and the adipose tissue lipidome
- PMID: 37115796
- PMCID: PMC10146461
- DOI: 10.1371/journal.pone.0284012
Sex-specific effects of CD248 on metabolism and the adipose tissue lipidome
Abstract
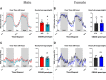
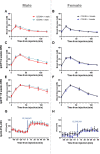
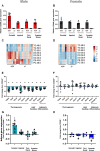
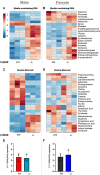
Cd248 has recently been associated with adipose tissue physiology, demonstrated by reduced weight gain in high fat diet-fed mice with genetic deletion of Cd248 relative to controls. Here we set out to determine the metabolic consequences of loss of Cd248. Strikingly, we find these to be sex specific; By subjecting Cd248-/- and Cd248+/+ mice to a high fat diet and indirect calorimetry study, we identified that only male Cd248-/- mice show reduced weight gain compared to littermate control wildtype mice. In addition, male (but not female) mice showed a lower respiratory exchange ratio on both chow and high fat diets, indicating a predisposition to metabolise lipid. Lipidomic studies on specific fat depots found reduced triglyceride and diglyceride deposition in male Cd248-/- mice, and this was supported by reduced expression of lipogenic and adipogenic genes. Finally, metabolomic analysis of isolated, differentiated preadipocytes found alterations in metabolic pathways associated with lipid deposition in cells isolated from male, but not female, Cd248-/- mice. Overall, our results highlight the importance of sex controls in animal studies and point to a role for Cd248 in sex- and depot-specific regulation of lipid metabolism.
Copyright: © 2023 Patrick et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: XT, MSG, LC, SH, DB, CC, GD are employees of AstraZeneca and may own stocks and/or restricted stocks. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

