Rapid Generation of Therapeutic Nanoparticles Using Cell-Free Expression Systems
- PMID: 37116099
- PMCID: PMC10611898
- DOI: 10.1002/smtd.202201718
Rapid Generation of Therapeutic Nanoparticles Using Cell-Free Expression Systems
Abstract
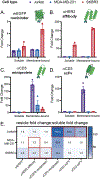
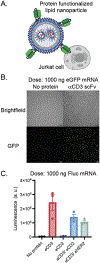
The surface modification of membrane-based nanoparticles, such as liposomes, polymersomes, and lipid nanoparticles, with targeting molecules, such as binding proteins, is an important step in the design of therapeutic materials. However, this modification can be costly and time-consuming, requiring cellular hosts for protein expression and lengthy purification and conjugation steps to attach proteins to the surface of nanocarriers, which ultimately limits the development of effective protein-conjugated nanocarriers. Here, the use of cell-free protein synthesis systems to rapidly create protein-conjugated membrane-based nanocarriers is demonstrated. Using this approach, multiple types of functional binding proteins, including affibodies, computationally designed proteins, and scFvs, can be cell-free expressed and conjugated to liposomes in one-pot. The technique can be expanded further to other nanoparticles, including polymersomes and lipid nanoparticles, and is amenable to multiple conjugation strategies, including surface attachment to and integration into nanoparticle membranes. Leveraging these methods, rapid design of bispecific artificial antigen presenting cells and enhanced delivery of lipid nanoparticle cargo in vitro is demonstrated. It is envisioned that this workflow will enable the rapid generation of membrane-based delivery systems and bolster our ability to create cell-mimetic therapeutics.
Keywords: cell-free expression; functionalization; lipid nanoparticles; liposomes; vesicles.
© 2023 Wiley-VCH GmbH.
Figures

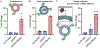

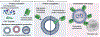
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

