Molecular mechanisms of stress-induced reactivation in mumps virus condensates
- PMID: 37116470
- PMCID: PMC10156176
- DOI: 10.1016/j.cell.2023.03.015
Molecular mechanisms of stress-induced reactivation in mumps virus condensates
Abstract
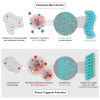
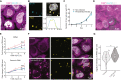
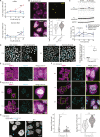
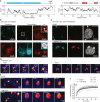
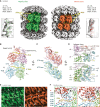
Negative-stranded RNA viruses can establish long-term persistent infection in the form of large intracellular inclusions in the human host and cause chronic diseases. Here, we uncover how cellular stress disrupts the metastable host-virus equilibrium in persistent infection and induces viral replication in a culture model of mumps virus. Using a combination of cell biology, whole-cell proteomics, and cryo-electron tomography, we show that persistent viral replication factories are dynamic condensates and identify the largely disordered viral phosphoprotein as a driver of their assembly. Upon stress, increased phosphorylation of the phosphoprotein at its interaction interface with the viral polymerase coincides with the formation of a stable replication complex. By obtaining atomic models for the authentic mumps virus nucleocapsid, we elucidate a concomitant conformational change that exposes the viral genome to its replication machinery. These events constitute a stress-mediated switch within viral condensates that provide an environment to support upregulation of viral replication.
Keywords: IDR; biomolecular condensates; cryo-electron tomography; cryo-focused ion beam; in-cell structural biology; intrinsically disordered regions; nucleocapsid; persistent infection; phosphorylation; viral replication; whole-cell proteomics.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.A.H. is a founder and scientific advisory board member of Dewpoint Therapeutics and a founder of Caraway Therapeutics. I.P. is an employee of Dewpoint Therapeutics.
Figures








Comment in
-
Stress-induced condensate switch awakens sleeping viruses.Cell Host Microbe. 2023 May 10;31(5):679-680. doi: 10.1016/j.chom.2023.04.008. Cell Host Microbe. 2023. PMID: 37167945
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

