Methotrexate suppresses psoriatic skin inflammation by inhibiting muropeptide transporter SLC46A2 activity
- PMID: 37116499
- PMCID: PMC10195032
- DOI: 10.1016/j.immuni.2023.04.001
Methotrexate suppresses psoriatic skin inflammation by inhibiting muropeptide transporter SLC46A2 activity
Abstract
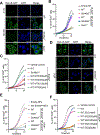
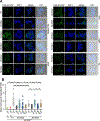
Cytosolic innate immune sensing is critical for protecting barrier tissues. NOD1 and NOD2 are cytosolic sensors of small peptidoglycan fragments (muropeptides) derived from the bacterial cell wall. These muropeptides enter cells, especially epithelial cells, through unclear mechanisms. We previously implicated SLC46 transporters in muropeptide transport in Drosophila immunity. Here, we focused on Slc46a2, which was highly expressed in mammalian epidermal keratinocytes, and showed that it was critical for the delivery of diaminopimelic acid (DAP)-muropeptides and activation of NOD1 in keratinocytes, whereas the related transporter Slc46a3 was critical for delivering the NOD2 ligand MDP to keratinocytes. In a mouse model, Slc46a2 and Nod1 deficiency strongly suppressed psoriatic inflammation, whereas methotrexate, a commonly used psoriasis therapeutic, inhibited Slc46a2-dependent transport of DAP-muropeptides. Collectively, these studies define SLC46A2 as a transporter of NOD1-activating muropeptides, with critical roles in the skin barrier, and identify this transporter as an important target for anti-inflammatory intervention.
Keywords: NOD1; SLC transporters; inflammation; innate immunity; methotrexate; muropeptide; psoriasis.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A provisional patent on targeting SLC46s to inhibit inflammation in psoriasis and other auto-inflammatory diseases has been filed by some of the authors (N.S., R.B., and M.H.O.). T.-D.K. consults for Pfizer.
Figures



Comment in
-
When SLC46A2 delivers cargo into keratinocytes.Immunity. 2023 May 9;56(5):897-900. doi: 10.1016/j.immuni.2023.04.008. Immunity. 2023. PMID: 37163988
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

