Structure and Function of Neuronal Circuits Linking Ventrolateral Preoptic Nucleus and Lateral Hypothalamic Area
- PMID: 37117013
- PMCID: PMC10255079
- DOI: 10.1523/JNEUROSCI.1913-22.2023
Structure and Function of Neuronal Circuits Linking Ventrolateral Preoptic Nucleus and Lateral Hypothalamic Area
Abstract
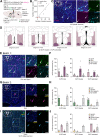
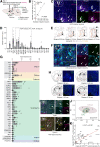
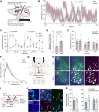
To understand how sleep-wakefulness cycles are regulated, it is essential to disentangle structural and functional relationships between the preoptic area (POA) and lateral hypothalamic area (LHA), since these regions play important yet opposing roles in the sleep-wakefulness regulation. GABA- and galanin (GAL)-producing neurons in the ventrolateral preoptic nucleus (VLPO) of the POA (VLPOGABA and VLPOGAL neurons) are responsible for the maintenance of sleep, while the LHA contains orexin-producing neurons (orexin neurons) that are crucial for maintenance of wakefulness. Through the use of rabies virus-mediated neural tracing combined with in situ hybridization (ISH) in male and female orexin-iCre mice, we revealed that the vesicular GABA transporter (Vgat, Slc32a1)- and galanin (Gal)-expressing neurons in the VLPO directly synapse with orexin neurons in the LHA. A majority (56.3 ± 8.1%) of all VLPO input neurons connecting to orexin neurons were double-positive for Vgat and Gal Using projection-specific rabies virus-mediated tracing in male and female Vgat-ires-Cre and Gal-Cre mice, we discovered that VLPOGABA and VLPOGAL neurons that send projections to the LHA received innervations from similarly distributed input neurons in many brain regions, with the POA and LHA being among the main upstream areas. Additionally, we found that acute optogenetic excitation of axons of VLPOGABA neurons, but not VLPOGAL neurons, in the LHA of male Vgat-ires-Cre mice induced wakefulness. This study deciphers the connectivity between the VLPO and LHA, provides a large-scale map of upstream neuronal populations of VLPO→LHA neurons, and reveals a previously uncovered function of the VLPOGABA→LHA pathway in the regulation of sleep and wakefulness.SIGNIFICANCE STATEMENT We identified neurons in the ventrolateral preoptic nucleus (VLPO) that are positive for vesicular GABA transporter (Vgat) and/or galanin (Gal) and serve as presynaptic partners of orexin-producing neurons in the lateral hypothalamic area (LHA). We depicted monosynaptic input neurons of GABA- and galanin-producing neurons in the VLPO that send projections to the LHA throughout the entire brain. Their input neurons largely overlap, suggesting that they comprise a common neuronal population. However, acute excitatory optogenetic manipulation of the VLPOGABA→LHA pathway, but not the VLPOGAL→LHA pathway, evoked wakefulness. This study shows the connectivity of major components of the sleep/wake circuitry in the hypothalamus and unveils a previously unrecognized function of the VLPOGABA→LHA pathway in sleep-wakefulness regulation. Furthermore, we suggest the existence of subpopulations of VLPOGABA neurons that innervate LHA.
Keywords: circuit; hypothalamus; preoptic area; sleep; wakefulness.
Copyright © 2023 the authors.
Figures




Similar articles
-
Monoamines Inhibit GABAergic Neurons in Ventrolateral Preoptic Area That Make Direct Synaptic Connections to Hypothalamic Arousal Neurons.J Neurosci. 2018 Jul 11;38(28):6366-6378. doi: 10.1523/JNEUROSCI.2835-17.2018. Epub 2018 Jun 18. J Neurosci. 2018. PMID: 29915137 Free PMC article.
-
RNA-seq analysis of galaninergic neurons from ventrolateral preoptic nucleus identifies expression changes between sleep and wake.BMC Genomics. 2020 Sep 14;21(1):633. doi: 10.1186/s12864-020-07050-7. BMC Genomics. 2020. PMID: 32928100 Free PMC article.
-
GABAergic neurons in the preoptic area send direct inhibitory projections to orexin neurons.Front Neural Circuits. 2013 Dec 2;7:192. doi: 10.3389/fncir.2013.00192. eCollection 2013. Front Neural Circuits. 2013. PMID: 24348342 Free PMC article.
-
Underlying brain mechanisms that regulate sleep-wakefulness cycles.Int Rev Neurobiol. 2010;93:1-21. doi: 10.1016/S0074-7742(10)93001-8. Int Rev Neurobiol. 2010. PMID: 20969999 Review.
-
Hypothalamic regulation of the sleep/wake cycle.Neurosci Res. 2017 May;118:74-81. doi: 10.1016/j.neures.2017.03.013. Epub 2017 May 17. Neurosci Res. 2017. PMID: 28526553 Review.
Cited by
-
Neural Network Mechanisms Underlying General Anesthesia: Cortical and Subcortical Nuclei.Neurosci Bull. 2024 Dec;40(12):1995-2011. doi: 10.1007/s12264-024-01286-z. Epub 2024 Aug 21. Neurosci Bull. 2024. PMID: 39168960 Review.
-
Lateral hypothalamic area high-frequency deep brain stimulation rescues memory decline in aged rat: behavioral, molecular, and electrophysiological study.Pflugers Arch. 2025 Mar;477(3):371-391. doi: 10.1007/s00424-024-03059-z. Epub 2025 Jan 21. Pflugers Arch. 2025. PMID: 39836224 Free PMC article.
-
Sleep need-dependent plasticity of a thalamic circuit promotes homeostatic recovery sleep.Science. 2025 Jun 19;388(6753):eadm8203. doi: 10.1126/science.adm8203. Epub 2025 Jun 19. Science. 2025. PMID: 40536979 Free PMC article.
-
Sleep Disorders: Pathogenesis and Therapeutic Interventions.MedComm (2020). 2025 Mar 10;6(3):e70130. doi: 10.1002/mco2.70130. eCollection 2025 Mar. MedComm (2020). 2025. PMID: 40066230 Free PMC article. Review.
-
GABALAGEN Facilitates Pentobarbital-Induced Sleep by Modulating the Serotonergic System in Rats.Curr Issues Mol Biol. 2024 Oct 4;46(10):11176-11189. doi: 10.3390/cimb46100663. Curr Issues Mol Biol. 2024. PMID: 39451543 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
