Repetitive administration of rituximab can achieve and maintain clinical remission in patients with MCD or FSGS
- PMID: 37117201
- PMCID: PMC10141841
- DOI: 10.1038/s41598-023-32576-7
Repetitive administration of rituximab can achieve and maintain clinical remission in patients with MCD or FSGS
Abstract
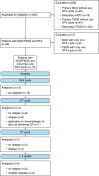
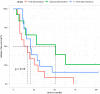
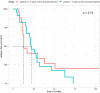
Minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS) are glomerulopathies associated with nephrotic syndrome. Primary forms of these diseases are treated with various regimes of immunosuppression. Frequently relapsing or glucocorticoid-dependent courses remain challenging. Here, a B-cell-depleting strategy with rituximab represents a salvage option although data are sparse in the adult population. In particular, there is limited evidence on the efficacy of restoring remission after initial successful treatment with rituximab and whether patients benefit from an individualized, relapse-based approach. We identified 13 patients who received multiple therapies with rituximab from the FOrMe-registry (NCT03949972), a nationwide registry for MCD and FSGS in Germany, or from the University Hospital of Cologne. Disease status, changes in serum creatinine, proteinuria, and time to relapse were evaluated. Relapse-free survival was compared to the patients' previous therapy regimens. Through all treatment cycles, an improvement of disease activity was shown leading to a complete remission in 72% and partial remission in 26% after 3 ([Formula: see text]0.001) and 6 months ([Formula: see text]0.001). Relapse-free survival increased from 4.5 months (95%-CI 3-10 months) to 21 months (95%-CI 16-32 months) ([Formula: see text]0.001) compared to previous immunosuppression regimens with no loss in estimated glomerular filtration over time (p = 0.53). Compared to continuous B-cell depletion, an individualized relapse-based approach led to a reduced rituximab exposure and significant cost savings. Relapse-based administration of rituximab in patients with MCD/FSGS with an initial good clinical response did not result in a decreased efficacy at a median follow-up duration of 110 months. Thus, reinduction therapies may provide an alternative to continuous B-cell-depletion and reduce the long-term side effects of continuous immunosuppression.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

