Wearable chemical sensors for biomarker discovery in the omics era
- PMID: 37117704
- PMCID: PMC9666953
- DOI: 10.1038/s41570-022-00439-w
Wearable chemical sensors for biomarker discovery in the omics era
Abstract
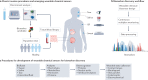
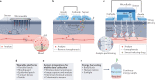
Biomarkers are crucial biological indicators in medical diagnostics and therapy. However, the process of biomarker discovery and validation is hindered by a lack of standardized protocols for analytical studies, storage and sample collection. Wearable chemical sensors provide a real-time, non-invasive alternative to typical laboratory blood analysis, and are an effective tool for exploring novel biomarkers in alternative body fluids, such as sweat, saliva, tears and interstitial fluid. These devices may enable remote at-home personalized health monitoring and substantially reduce the healthcare costs. This Review introduces criteria, strategies and technologies involved in biomarker discovery using wearable chemical sensors. Electrochemical and optical detection techniques are discussed, along with the materials and system-level considerations for wearable chemical sensors. Lastly, this Review describes how the large sets of temporal data collected by wearable sensors, coupled with modern data analysis approaches, would open the door for discovering new biomarkers towards precision medicine.
© 2022. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Zhao J, Guo H, Li J, Bandodkar AJ, Rogers JA. Body-interfaced chemical sensors for noninvasive monitoring and analysis of biofluids. Trends Chem. 2019;1:559–571. doi: 10.1016/j.trechm.2019.07.001. - DOI

