Intracrine activity involving NAD-dependent circadian steroidogenic activity governs age-associated meibomian gland dysfunction
- PMID: 37117756
- PMCID: PMC10154200
- DOI: 10.1038/s43587-021-00167-8
Intracrine activity involving NAD-dependent circadian steroidogenic activity governs age-associated meibomian gland dysfunction
Abstract
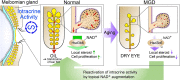
Canonically, hormones are produced in the endocrine organs and delivered to target tissues. However, for steroids, the concept of tissue intracrinology, whereby hormones are produced in the tissues where they exert their effect without release into circulation, has been proposed, but its role in physiology/disease remains unclear. The meibomian glands in the eyelids produce oil to prevent tear evaporation, which reduces with aging. Here, we demonstrate that (re)activation of local intracrine activity through nicotinamide adenine dinucleotide (NAD+)-dependent circadian 3β-hydroxyl-steroid dehydrogenase (3β-HSD) activity ameliorates age-associated meibomian gland dysfunction and accompanying evaporative dry eye disease. Genetic ablation of 3β-HSD nullified local steroidogenesis and led to atrophy of the meibomian gland. Conversely, reactivation of 3β-HSD activity by boosting its coenzyme NAD+ availability improved glandular cell proliferation and alleviated the dry eye disease phenotype. Both women and men express 3β-HSD in the meibomian gland. Enhancing local steroidogenesis may help combat age-associated meibomian gland dysfunction.
© 2022. The Author(s).
Conflict of interest statement
M.D., T.N. and M.M. have filed a patent application related to the new uses of NMN and NR (WO2020262497). M.D., T.S. and S.K. received institutional research funding from Senju Pharmaceutical Co. T.N. and M.M. are employees of Senju Pharmaceutical Co. All other authors declare no competing interests.
Figures







Comment in
-
NAD+ boosting brings tears to aging eyes.Nat Aging. 2022 Feb;2(2):97-99. doi: 10.1038/s43587-022-00172-5. Nat Aging. 2022. PMID: 37117757 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

