An IL1RL1 genetic variant lowers soluble ST2 levels and the risk effects of APOE-ε4 in female patients with Alzheimer's disease
- PMID: 37117777
- PMCID: PMC10154240
- DOI: 10.1038/s43587-022-00241-9
An IL1RL1 genetic variant lowers soluble ST2 levels and the risk effects of APOE-ε4 in female patients with Alzheimer's disease
Abstract
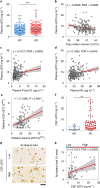
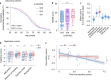
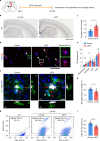
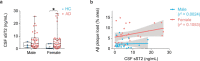
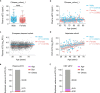
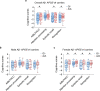
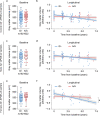
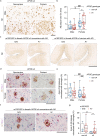
Changes in the levels of circulating proteins are associated with Alzheimer's disease (AD), whereas their pathogenic roles in AD are unclear. Here, we identified soluble ST2 (sST2), a decoy receptor of interleukin-33-ST2 signaling, as a new disease-causing factor in AD. Increased circulating sST2 level is associated with more severe pathological changes in female individuals with AD. Genome-wide association analysis and CRISPR-Cas9 genome editing identified rs1921622 , a genetic variant in an enhancer element of IL1RL1, which downregulates gene and protein levels of sST2. Mendelian randomization analysis using genetic variants, including rs1921622 , demonstrated that decreased sST2 levels lower AD risk and related endophenotypes in females carrying the Apolipoprotein E (APOE)-ε4 genotype; the association is stronger in Chinese than in European-descent populations. Human and mouse transcriptome and immunohistochemical studies showed that rs1921622 /sST2 regulates amyloid-beta (Aβ) pathology through the modulation of microglial activation and Aβ clearance. These findings demonstrate how sST2 level is modulated by a genetic variation and plays a disease-causing role in females with AD.
© 2022. The Author(s).
Conflict of interest statement
All authors declare no competing interests.
Figures






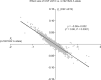

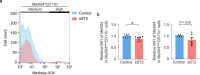
Comment in
-
Reducing decoys focuses fighting microglia.Nat Aging. 2022 Jul;2(7):573-575. doi: 10.1038/s43587-022-00251-7. Epub 2022 Jul 15. Nat Aging. 2022. PMID: 36793992 Free PMC article.
References
-
- Alzheimer’s Association Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15:321–387.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

