CSF tau phosphorylation occupancies at T217 and T205 represent improved biomarkers of amyloid and tau pathology in Alzheimer's disease
- PMID: 37117788
- PMCID: PMC10154225
- DOI: 10.1038/s43587-023-00380-7
CSF tau phosphorylation occupancies at T217 and T205 represent improved biomarkers of amyloid and tau pathology in Alzheimer's disease
Abstract
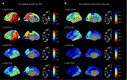
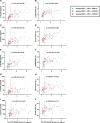
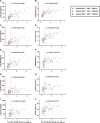
Cerebrospinal fluid (CSF) amyloid-β peptide (Aβ)42/Aβ40 and the concentration of tau phosphorylated at site 181 (p-tau181) are well-established biomarkers of Alzheimer's disease (AD). The present study used mass spectrometry to measure concentrations of nine phosphorylated and five nonphosphorylated tau species and phosphorylation occupancies (percentage phosphorylated/nonphosphorylated) at ten sites. In the present study we show that, in 750 individuals with a median age of 71.2 years, CSF pT217/T217 predicted the presence of brain amyloid by positron emission tomography (PET) slightly better than Aβ42/Aβ40 (P = 0.02). Furthermore, for individuals with positive brain amyloid by PET (n = 263), CSF pT217/T217 was more strongly correlated with the amount of amyloid (Spearman's ρ = 0.69) than Aβ42/Aβ40 (ρ = -0.42, P < 0.0001). In two independent cohorts of participants with symptoms of AD dementia (n = 55 and n = 90), CSF pT217/T217 and pT205/T205 were better correlated with tau PET measures than CSF p-tau181 concentration. These findings suggest that CSF pT217/T217 and pT205/T205 represent improved CSF biomarkers of amyloid and tau pathology in AD.
© 2023. The Author(s).
Conflict of interest statement
N.R.B., K.H., C.S., V.O. and R.J.B. are coinventors on the following US patent applications: ‘Methods to detect novel tau species in CSF and use thereof to track tau neuropathology in Alzheimer’s disease and other tauopathies’ (PCT/US2020/046224, N.R.B., K.H., C.S. and R.J.B.); ‘CSF phosphorylated tau and amyloid beta profiles as biomarkers of tauopathies’ (PCT/US2022/022906, N.R.B., C.S. and R.J.B.); ‘Plasma based methods for detecting CNS amyloid deposition’ (PCT/UC2018/030518, V.O. and R.J.B.); and ‘Methods of diagnosing and treating based on site-specific tau phosphorylation’ (PCT/US2019/030725, N.R.B. and R.J.B.). N.R.B., K.H., C.S., V.O. and R.J.B. may receive a royalty income based on technology licensed by Washington University to C2N Diagnostics. K.H. is an Eisai-sponsored voluntary research associate professor at Washington University and has received a salary from Eisai. A.M.F. has received research funding from Biogen, Centene, Fujirebio and Roche Diagnostics. She is a member of the scientific advisory boards for Roche Diagnostics, Genentech and Diadem. She consults for DiamiR and Seimens Healthcare Diagnostics Inc. T.L.S.B. has investigator-initiated research funding from the NIH, the Alzheimer’s Association, the Barnes-Jewish Hospital Foundation and Siemens. She participates as a site investigator in clinical trials sponsored by Avid Radiopharmaceuticals, Eli Lilly, Biogen, Eisai, Janssen and Roche, and serves as a consultant to Biogen, Eli Lilly, Eisai and Siemens. C.X. consulted for DIADEM and has used funding from the NIH to hire C2N Diagnostics as a vendor in another independent NIH-funded project. He received no funding from C2N Diagnostics. Neither J.C.M. nor his family owns stock or has equity interest (outside of mutual funds or other externally directed accounts) in any pharmaceutical or biotechnology company. O.H. has acquired research support (for the institution) from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer and Roche. In the past 2 years, he has received consultancy/speaker fees from AC Immune, Amylyx, Alzpath, BioArctic, Biogen, Cerveau, Eisai, Fujirebio, Genentech, Novartis, Novo Nordisk, Roche and Siemens. R.J.B. cofounded C2N Diagnostics. Washington University and R.J.B. have equity ownership interest in C2N Diagnostics and receive a royalty income based on technology (stable isotope labeling kinetics, blood plasma assay and methods of diagnosing AD with phosphorylation changes) licensed by Washington University to C2N Diagnostics. R.J.B. receives an income from C2N Diagnostics for serving on the scientific advisory board, and has received research funding from Avid Radiopharmaceuticals, Janssen, Roche/Genentech, Eli Lilly, Eisai, Biogen, AbbVie, Bristol Myers Squibb and Novartis. S.E.S. has analyzed data provided by C2N Diagnostics to Washington University, but she has not received any research funding or personal compensation from C2N Diagnostics or any other for-profit organizations. The remaining authors declare no competing interests.
Figures






Similar articles
-
MAPT R406W increases tau T217 phosphorylation in absence of amyloid pathology.Ann Clin Transl Neurol. 2021 Sep;8(9):1817-1830. doi: 10.1002/acn3.51435. Epub 2021 Aug 2. Ann Clin Transl Neurol. 2021. PMID: 34342183 Free PMC article.
-
Predicting continuous amyloid PET values with CSF tau phosphorylation occupancies.Alzheimers Dement. 2024 Sep;20(9):6365-6373. doi: 10.1002/alz.14132. Epub 2024 Jul 23. Alzheimers Dement. 2024. PMID: 39041391 Free PMC article.
-
Correlation between Cerebrospinal Fluid Core Alzheimer's Disease Biomarkers and β-Amyloid PET in Chinese Dementia Population.ACS Chem Neurosci. 2022 May 18;13(10):1558-1565. doi: 10.1021/acschemneuro.2c00120. Epub 2022 Apr 27. ACS Chem Neurosci. 2022. PMID: 35476397
-
Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer's Disease.Alzheimers Res Ther. 2019 Apr 22;11(1):34. doi: 10.1186/s13195-019-0485-0. Alzheimers Res Ther. 2019. PMID: 31010420 Free PMC article. Review.
-
Predictive Accuracy of Blood-Derived Biomarkers for Amyloid-β Brain Deposition Along with the Alzheimer's Disease Continuum: A Systematic Review.J Alzheimers Dis. 2021;84(1):393-407. doi: 10.3233/JAD-210496. J Alzheimers Dis. 2021. PMID: 34542072
Cited by
-
The Relationship between p-tau217, p-tau231, and p-tau205 in the Human Brain Is Affected by the Cellular Environment and Alzheimer's Disease Pathology.Cells. 2024 Feb 11;13(4):331. doi: 10.3390/cells13040331. Cells. 2024. PMID: 38391945 Free PMC article.
-
Cognition Mediates the Association Between Cerebrospinal Fluid Biomarkers of Amyloid and P-Tau and Neuropsychiatric Symptoms.J Alzheimers Dis. 2024;100(3):1055-1073. doi: 10.3233/JAD-240125. J Alzheimers Dis. 2024. PMID: 38995786 Free PMC article.
-
Temporal Dynamics and Biological Variability of Alzheimer Biomarkers.JAMA Neurol. 2025 Feb 17;82(4):384-96. doi: 10.1001/jamaneurol.2024.5263. Online ahead of print. JAMA Neurol. 2025. PMID: 39960728
-
Brain vasculature accumulates tau and is spatially related to tau tangle pathology in Alzheimer's disease.Acta Neuropathol. 2024 Jun 17;147(1):101. doi: 10.1007/s00401-024-02751-9. Acta Neuropathol. 2024. PMID: 38884806 Free PMC article.
-
Clinical criteria for limbic-predominant age-related TDP-43 encephalopathy.Alzheimers Dement. 2025 Jan;21(1):e14202. doi: 10.1002/alz.14202. Epub 2025 Jan 14. Alzheimers Dement. 2025. PMID: 39807681 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

