Immune system-wide Mendelian randomization and triangulation analyses support autoimmunity as a modifiable component in dementia-causing diseases
- PMID: 37118290
- PMCID: PMC10154235
- DOI: 10.1038/s43587-022-00293-x
Immune system-wide Mendelian randomization and triangulation analyses support autoimmunity as a modifiable component in dementia-causing diseases
Abstract
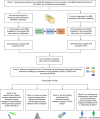
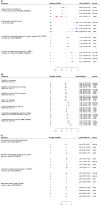
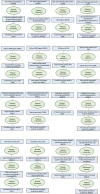
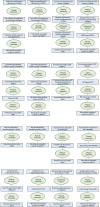
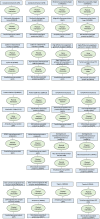
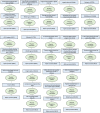
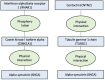
Immune system and blood-brain barrier dysfunction are implicated in the development of Alzheimer's and other dementia-causing diseases, but their causal role remains unknown. We performed Mendelian randomization for 1,827 immune system- and blood-brain barrier-related biomarkers and identified 127 potential causal risk factors for dementia-causing diseases. Pathway analyses linked these biomarkers to amyloid-β, tau and α-synuclein pathways and to autoimmunity-related processes. A phenome-wide analysis using Mendelian randomization-based polygenic risk score in the FinnGen study (n = 339,233) for the biomarkers indicated shared genetic background for dementias and autoimmune diseases. This association was further supported by human leukocyte antigen analyses. In inverse-probability-weighted analyses that simulate randomized controlled drug trials in observational data, anti-inflammatory methotrexate treatment reduced the incidence of Alzheimer's disease in high-risk individuals (hazard ratio compared with no treatment, 0.64, 95% confidence interval 0.49-0.88, P = 0.005). These converging results from different lines of human research suggest that autoimmunity is a modifiable component in dementia-causing diseases.
© 2022. The Author(s).
Conflict of interest statement
H.R. is a full-time employee at Biogen. Biogen had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The remaining authors declare no competing interests.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

