Type 2 Diabetes (T2DM) and Parkinson's Disease (PD): a Mechanistic Approach
- PMID: 37118323
- PMCID: PMC10144908
- DOI: 10.1007/s12035-023-03359-y
Type 2 Diabetes (T2DM) and Parkinson's Disease (PD): a Mechanistic Approach
Abstract
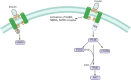
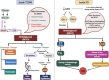
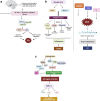
Growing evidence suggest that there is a connection between Parkinson's disease (PD) and insulin dysregulation in the brain, whilst the connection between PD and type 2 diabetes mellitus (T2DM) is still up for debate. Insulin is widely recognised to play a crucial role in neuronal survival and brain function; any changes in insulin metabolism and signalling in the central nervous system (CNS) can lead to the development of various brain disorders. There is accumulating evidence linking T2DM to PD and other neurodegenerative diseases. In fact, they have a lot in common patho-physiologically, including insulin dysregulation, oxidative stress resulting in mitochondrial dysfunction, microglial activation, and inflammation. As a result, initial research should focus on the role of insulin and its molecular mechanism in order to develop therapeutic outcomes. In this current review, we will look into the link between T2DM and PD, the function of insulin in the brain, and studies related to impact of insulin in causing T2DM and PD. Further, we have also highlighted the role of various insulin signalling pathway in both T2DM and PD. We have also suggested that T2DM-targeting pharmacological strategies as potential therapeutic approach for individuals with cognitive impairment, and we have demonstrated the effectiveness of T2DM-prescribed drugs through current PD treatment trials. In conclusion, this investigation would fill a research gap in T2DM-associated Parkinson's disease (PD) with a potential therapy option.
Keywords: Insulin; Parkinson’s disease; Pathophysiology; Therapeutics; Type 2 diabetes mellitus.
© 2023. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Venkatesan D, Iyer M, Krishnan P, Wilson RS, Vellingiri B. A late-onset Parkinson’s disease in tribes in India – a case report. Brain Disord. 2021;3:100015. doi: 10.1016/j.dscb.2021.100015. - DOI
-
- Venkatesan D, Iyer M, Wilson R, Lakshmipathy G, Vellingiri B. The association between multiple risk factors, clinical correlations and molecular insights in Parkinson’s disease patients from Tamil Nadu population, India. Neurosci Lett. 2021;755:135903. doi: 10.1016/j.neulet.2021.135903. - DOI - PubMed
-
- M. Iyer et al., “Role of RhoA-ROCK signaling in Parkinson’s disease,” European Journal of Pharmacology, vol. 894, p. 173815, Mar. 2021, 10.1016/j.ejphar.2020.173815. - PubMed
-
- B. Vellingiri et al (2022) “Influence of heavy metals in Parkinson’s disease: an overview,” J Neurol10.1007/s00415-022-11282-w - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

