Biomarker modeling of Alzheimer's disease using PET-based Braak staging
- PMID: 37118445
- PMCID: PMC10154209
- DOI: 10.1038/s43587-022-00204-0
Biomarker modeling of Alzheimer's disease using PET-based Braak staging
Abstract
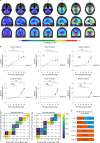
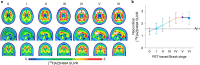
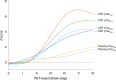
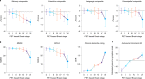
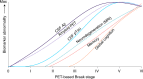
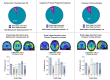
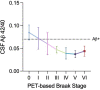
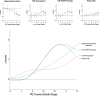
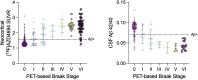
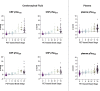
Gold-standard diagnosis of Alzheimer's disease (AD) relies on histopathological staging systems. Using the topographical information from [18F]MK6240 tau positron-emission tomography (PET), we applied the Braak tau staging system to 324 living individuals. We used PET-based Braak stage to model the trajectories of amyloid-β, phosphorylated tau (pTau) in cerebrospinal fluid (pTau181, pTau217, pTau231 and pTau235) and plasma (pTau181 and pTau231), neurodegeneration and cognitive symptoms. We identified nonlinear AD biomarker trajectories corresponding to the spatial extent of tau-PET, with modest biomarker changes detectable by Braak stage II and significant changes occurring at stages III-IV, followed by plateaus. Early Braak stages were associated with isolated memory impairment, whereas Braak stages V-VI were incompatible with normal cognition. In 159 individuals with follow-up tau-PET, progression beyond stage III took place uniquely in the presence of amyloid-β positivity. Our findings support PET-based Braak staging as a framework to model the natural history of AD and monitor AD severity in living humans.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Comment in
-
Imaging Alzheimer's pathology stage by stage.Nat Aging. 2022 Jun;2(6):465-467. doi: 10.1038/s43587-022-00236-6. Nat Aging. 2022. PMID: 37118453 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

