A linked physiologically based pharmacokinetic model for hydroxychloroquine and metabolite desethylhydroxychloroquine in SARS-CoV-2(-)/(+) populations
- PMID: 37118968
- PMCID: PMC10339702
- DOI: 10.1111/cts.13527
A linked physiologically based pharmacokinetic model for hydroxychloroquine and metabolite desethylhydroxychloroquine in SARS-CoV-2(-)/(+) populations
Abstract
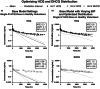
Hydroxychloroquine (HCQ) is Food and Drug Administration (FDA)-approved for malaria, systemic and chronic discoid lupus erythematosus, and rheumatoid arthritis. Because HCQ has a proposed multimodal mechanism of action and a well-established safety profile, it is often investigated as a repurposed therapeutic for a range of indications. There is a large degree of uncertainty in HCQ pharmacokinetic (PK) parameters which complicates dose selection when investigating its use in new disease states. Complications with HCQ dose selection emerged as multiple clinical trials investigated HCQ as a potential therapeutic in the early stages of the COVID-19 pandemic. In addition to uncertainty in baseline HCQ PK parameters, it was not clear if disease-related consequences of SARS-CoV-2 infection/COVID-19 would be expected to impact the PK of HCQ and its primary metabolite desethylhydroxychloroquine (DHCQ). To address the question whether SARS-CoV-2 infection/COVID-19 impacted HCQ and DHCQ PK, dried blood spot samples were collected from SARS-CoV-2(-)/(+) participants administered HCQ. When a previously published physiologically based pharmacokinetic (PBPK) model was used to fit the data, the variability in exposure of HCQ and DHCQ was not adequately captured and DHCQ concentrations were overestimated. Improvements to the previous PBPK model were made by incorporating the known range of blood to plasma concentration ratios (B/P) for each compound, adjusting HCQ and DHCQ distribution settings, and optimizing DHCQ clearance. The final PBPK model adequately captured the HCQ and DHCQ concentrations observed in SARS-CoV-2(-)/(+)participants, and incorporating COVID-19-associated changes in cytochrome P450 activity did not further improve model performance for the SARS-CoV-2(+) population.
© 2023 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
A.B. reports research support from the US National Institutes of Health, the Bill and Melinda Gates Foundation, the Foundation for Innovative New Diagnostics, and the New York City Department of Health and Mental Hygiene, and compensation for consulting services from Gates Ventures. C.J. reports consultancies to AbbVie, GSK, and Gilead, and receives research support from Gilead and royalties from UpToDate. R.V.B. reports Regeneron Pharmaceuticals provided conference abstract and manuscript writing support outside the submitted work. R.L. serves on the Scientific Advisory Board for Gilead and Merck in addition to reporting consultancy agreements for Cepheid and Janssen. All the other authors declared no competing interests for this work.
Figures




References
-
- Concordia . PLAQUENIL (hydroxychloroquine) [product label]. 2021. Accessed December 20, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/009768s053lbl.pdf
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

