Low-Temperature Gas-Phase Kinetics of Ethanol-Methanol Heterodimer Formation
- PMID: 37119198
- PMCID: PMC10184117
- DOI: 10.1021/acs.jpca.3c01312
Low-Temperature Gas-Phase Kinetics of Ethanol-Methanol Heterodimer Formation
Abstract
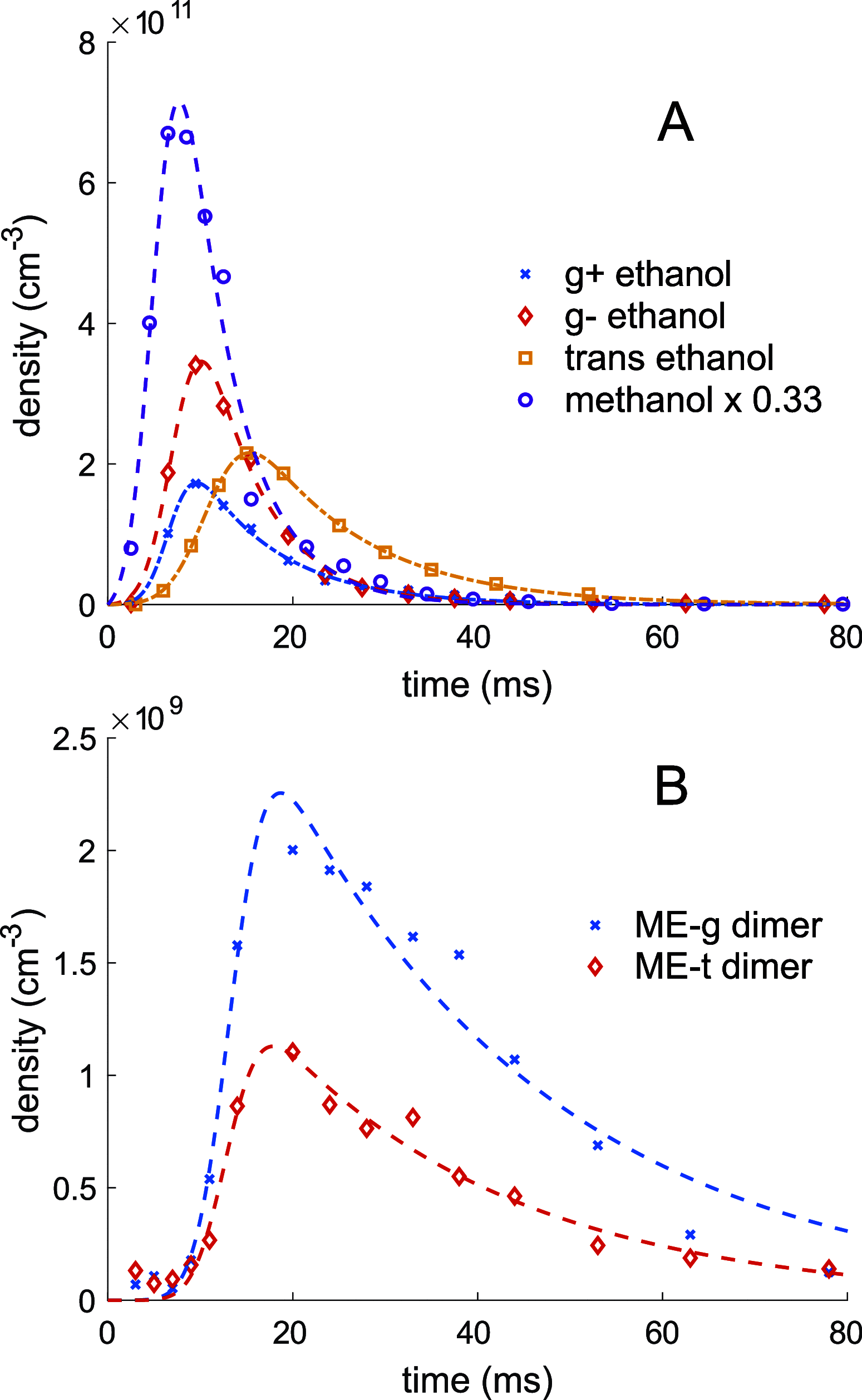
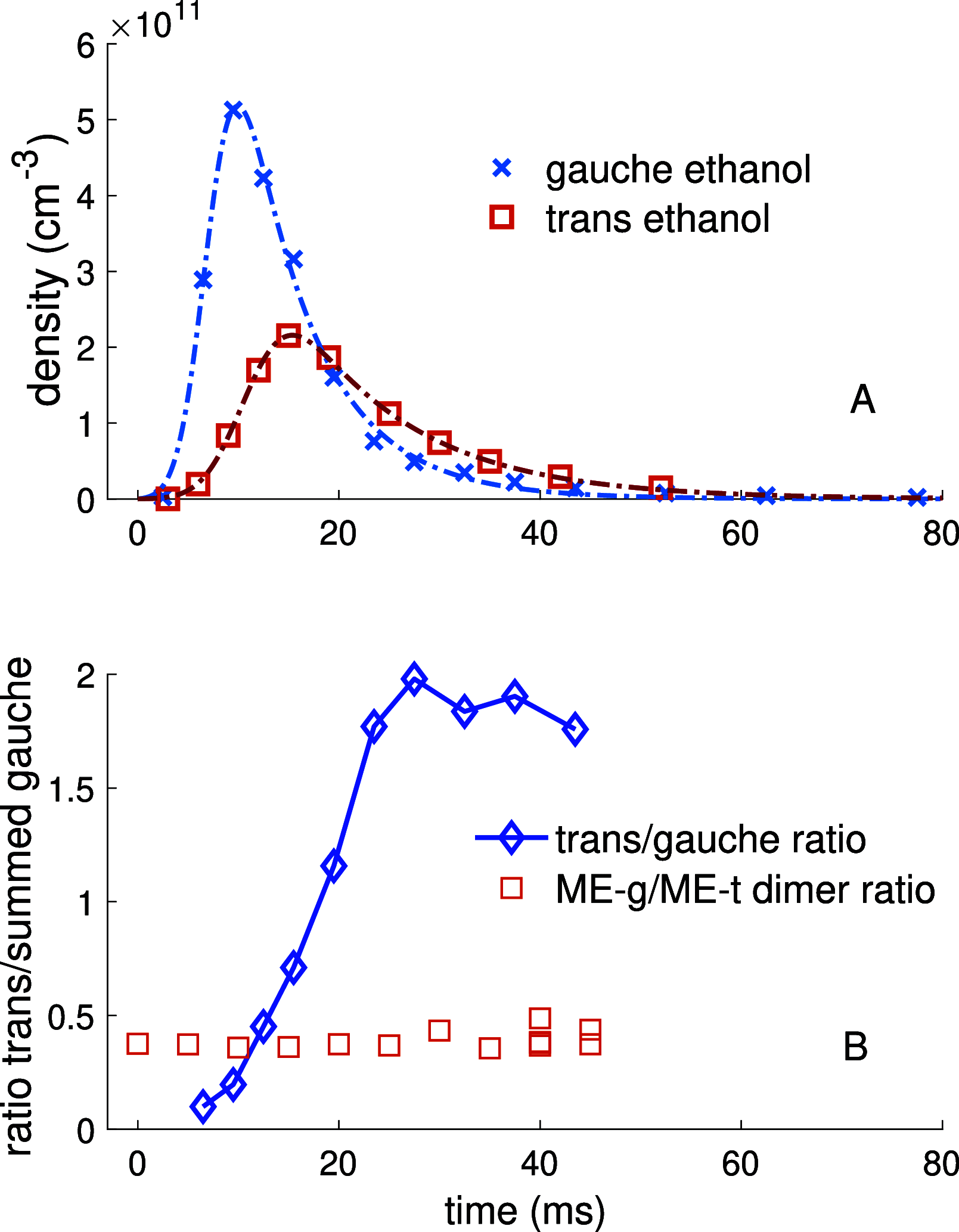
The structures of gas-phase noncovalently bound clusters have long been studied in supersonic expansions. This method of study, while providing a wealth of information about the nature of noncovalent bonds, precludes observation of the formation of the cluster, as the clusters form just after the orifice of the pulsed valve. Here, we directly observe formation of ethanol-methanol dimers via microwave spectroscopy in a controlled cryogenic environment. Time profiles of the concentration of reagents in the cell yielded gas-phase reaction rate constants of kMe-g = (2.8 ± 1.4) × 10-13 cm3 molecule-1 s-1 and kMe-t = (1.6 ± 0.8) × 10-13 cm3 molecule-1 s-1 for the pseudo-second-order ethanol-methanol dimerization reaction at 8 K. The relaxation cross section between the gauche and trans conformers of ethanol was also measured using the same technique. In addition, thermodynamic relaxation between conformers of ethanol over time allowed for selection of conformer stoichiometry in the ethanol-methanol dimerization reaction, but no change in the ratio of dimer conformers was observed with changing ethanol monomer stoichiometry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Thomas M.; Suarez-Martinez I.; Yu L.-J.; Karton A.; Chandler G. S.; Robinson M.; Cherchneff I.; Talbi D.; Spagnoli D. Atomistic simulations of the aggregation of small aromatic molecules in homogenous and heterogenous mixtures. Phys. Chem. Chem. Phys. 2020, 22, 21005–21014. 10.1039/D0CP02622K. - DOI - PMC - PubMed
-
- Stein T.; Jose J. Molecular formation upon ionization of van der Waals clusters and implication to astrochemistry. Isr. J. Chem. 2020, 60, 842–849. 10.1002/ijch.201900127. - DOI
LinkOut - more resources
Full Text Sources

