CSF neopterin, quinolinic acid and kynurenine/tryptophan ratio are biomarkers of active neuroinflammation
- PMID: 37119734
- PMCID: PMC10165192
- DOI: 10.1016/j.ebiom.2023.104589
CSF neopterin, quinolinic acid and kynurenine/tryptophan ratio are biomarkers of active neuroinflammation
Abstract
Background: Defining the presence of acute and chronic brain inflammation remains a challenge to clinicians due to the heterogeneity of clinical presentations and aetiologies. However, defining the presence of neuroinflammation, and monitoring the effects of therapy is important given its reversible and potentially damaging nature. We investigated the utility of CSF metabolites in the diagnosis of primary neuroinflammatory disorders such as encephalitis and explored the potential pathogenic role of inflammation in epilepsy.
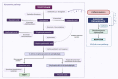
Methods: Cerebrospinal fluid (CSF) collected from 341 paediatric patients (169 males, median age 5.8 years, range 0.1-17.1) were examined. The patients were separated into a primary inflammatory disorder group (n = 90) and epilepsy group (n = 80), who were compared with three control groups including neurogenetic and structural (n = 76), neurodevelopmental disorders, psychiatric and functional neurological disorders (n = 63), and headache (n = 32).
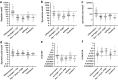
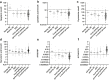
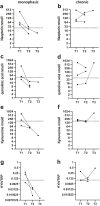
Findings: There were statistically significant increases of CSF neopterin, kynurenine, quinolinic acid and kynurenine/tryptophan ratio (KYN/TRP) in the inflammation group compared to all control groups (all p < 0.0003). As biomarkers, at thresholds with 95% specificity, CSF neopterin had the best sensitivity for defining neuroinflammation (82%, CI 73-89), then quinolinic acid (57%, CI 47-67), KYN/TRP ratio (47%, CI 36-56) and kynurenine (37%, CI 28-48). CSF pleocytosis had sensitivity of 53%, CI 42-64). The area under the receiver operating characteristic curve (ROC AUC) of CSF neopterin (94.4% CI 91.0-97.7%) was superior to that of CSF pleocytosis (84.9% CI 79.5-90.4%) (p = 0.005). CSF kynurenic acid/kynurenine ratio (KYNA/KYN) was statistically decreased in the epilepsy group compared to all control groups (all p ≤ 0.0003), which was evident in most epilepsy subgroups.
Interpretation: Here we show that CSF neopterin, kynurenine, quinolinic acid and KYN/TRP are useful diagnostic and monitoring biomarkers of neuroinflammation. These findings provide biological insights into the role of inflammatory metabolism in neurological disorders and provide diagnostic and therapeutic opportunities for improved management of neurological diseases.
Funding: Financial support for the study was granted by Dale NHMRC Investigator grant APP1193648, University of Sydney, Petre Foundation, Cerebral Palsy Alliance and Department of Biochemistry at the Children's Hospital at Westmead. Prof Guillemin is funded by NHMRC Investigator grant APP 1176660 and Macquarie University.
Keywords: Cerebrospinal fluid metabolomics; Encephalitis; Epilepsy; Kynurenine pathway; Neopterin; Neurodevelopmental disorders.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests M Farrar reports grants from NHMRC and Cerebral Palsy Alliance Research Foundation, honoraria for educational presentations from Roche, Biogen and Novartis, participation on advisory board for Novartis Gene therapies and Roche, being medical director for Muscular dystrophy NSW and member of scientific and medical committee of Childhood dementia and Friedreich's ataxia. R Dale reports honorarium from Beijing pediatric neurology conference. The other authors have declared that no conflict of interest exists.
Figures

References
-
- Fung A., Vizcaychipi M., Lloyd D., Wan Y., Ma D. Central nervous system inflammation in disease related conditions: mechanistic prospects. Brain Res. 2012;1446:144–155. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

