The interaction between autophagy, Helicobacter pylori, and gut microbiota in gastric carcinogenesis
- PMID: 37120362
- PMCID: PMC10523907
- DOI: 10.1016/j.tim.2023.04.001
The interaction between autophagy, Helicobacter pylori, and gut microbiota in gastric carcinogenesis
Abstract
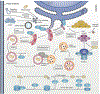
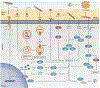
Chronic infection with Helicobacter pylori is the primary risk factor for the development of gastric cancer. Hindering our ability to comprehend the precise role of autophagy during H. pylori infection is the complexity of context-dependent autophagy signaling pathways. Recent and ongoing progress in understanding H. pylori virulence allows new frontiers of research for the crosstalk between autophagy and H. pylori. Novel approaches toward discovering autophagy signaling networks have further revealed their critical influence on the structure of gut microbiota and the metabolome. Here we intend to present a holistic view of the perplexing role of autophagy in H. pylori pathogenesis and carcinogenesis. We also discuss the intermediate role of autophagy in H. pylori-mediated modification of gut inflammatory responses and microbiota structure.
Keywords: Helicobacter pylori; anticancer therapy; autophagy; gastric cancer; gut microbiota.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests There are no interests to declare.
Figures



References
-
- Wang L, et al. (2023) The emerging mechanisms and functions of microautophagy. Nature Reviews Molecular Cell Biology 24, 186–203 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

