Piezo2 expressing nociceptors mediate mechanical sensitization in experimental osteoarthritis
- PMID: 37120427
- PMCID: PMC10148822
- DOI: 10.1038/s41467-023-38241-x
Piezo2 expressing nociceptors mediate mechanical sensitization in experimental osteoarthritis
Abstract
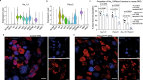
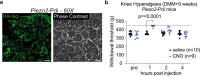
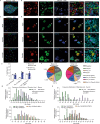
Non-opioid targets are needed for addressing osteoarthritis pain, which is mechanical in nature and associated with daily activities such as walking and climbing stairs. Piezo2 has been implicated in the development of mechanical pain, but the mechanisms by which this occurs remain poorly understood, including the role of nociceptors. Here we show that nociceptor-specific Piezo2 conditional knock-out mice were protected from mechanical sensitization associated with inflammatory joint pain in female mice, joint pain associated with osteoarthritis in male mice, as well as both knee swelling and joint pain associated with repeated intra-articular injection of nerve growth factor in male mice. Single cell RNA sequencing of mouse lumbar dorsal root ganglia and in situ hybridization of mouse and human lumbar dorsal root ganglia revealed that a subset of nociceptors co-express Piezo2 and Ntrk1 (the gene that encodes the nerve growth factor receptor TrkA). These results suggest that nerve growth factor-mediated sensitization of joint nociceptors, which is critical for osteoarthritic pain, is also dependent on Piezo2, and targeting Piezo2 may represent a therapeutic option for osteoarthritis pain control.
© 2023. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: A.M. M. is a consultant of Asahi Kasei Pharma and a consultant of 23andMe. The remaining authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

