A Phase 1/2 trial of SRA737 (a Chk1 inhibitor) administered orally in patients with advanced cancer
- PMID: 37120671
- PMCID: PMC10307885
- DOI: 10.1038/s41416-023-02279-x
A Phase 1/2 trial of SRA737 (a Chk1 inhibitor) administered orally in patients with advanced cancer
Abstract
Background: This was a first-in-human Phase 1/2 open-label dose-escalation study of the novel checkpoint kinase 1 (Chk1) inhibitor SRA737.
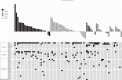
Methods: Patients with advanced solid tumours enrolled in dose-escalation cohorts and received SRA737 monotherapy orally on a continuous daily (QD) dosing schedule in 28-day cycles. Expansion cohorts included up to 20 patients with prospectively selected, pre-specified response predictive biomarkers.
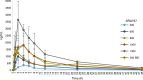
Results: In total, 107 patients were treated at dose levels from 20-1300 mg. The maximum tolerated dose (MTD) of SRA737 was 1000 mg QD, the recommended Phase 2 dose (RP2D) was 800 mg QD. Common toxicities of diarrhoea, nausea and vomiting were generally mild to moderate. Dose-limiting toxicity at daily doses of 1000 and 1300 mg QD SRA737 included gastrointestinal events, neutropenia and thrombocytopenia. Pharmacokinetic analysis at the 800 mg QD dose showed a mean Cmin of 312 ng/mL (546 nM), exceeding levels required to cause growth delay in xenograft models. No partial or complete responses were seen.
Conclusions: SRA737 was well tolerated at doses that achieved preclinically relevant drug concentrations but single agent activity did not warrant further development as monotherapy. Given its mechanism of action resulting in abrogating DNA damage repair, further clinical development of SRA737 should be as combination therapy.
Clinical trial registration: Clinicaltrials.gov NCT02797964.
© 2023. The Author(s).
Conflict of interest statement
SRA737 was discovered in collaboration with The Institute of Cancer Research, London, UK (ICR). UB is an employee of ICR but does not receive royalties for discovery of SRA737. UB has spoken at Sierra Oncology and AVACTA advisory boards (non-remunerated) and has received honoraria from Pegasys, Boehringer Ingelheim, Idea Pharma, Novartis, and Karus Therapeutics unrelated to the presented work. He has received research funding from Avacta, Verastem Oncology, Chugai, Carrick Therapeutics, BTG, and Onyx all unrelated to this work. Evans is Clinical Subjects Editor, British Journal of Cancer. SB has received research funding from Nucana PLC, Astex, Incyte, Tesaro, Redx, MSD, Roche, UCB and consulting fees from Ellipses and Theolytics, unrelated to this work.
Figures

References
-
- Osborne JD, Matthews TP, McHardy T, Proisy N, Cheung KM, Lainchbury M, et al. Multiparameter lead optimization to give an oral checkpoint kinase 1 (CHK1) inhibitor clinical candidate: (R)-5-((4-((morpholin-2-ylmethyl)amino)-5-(trifluoromethyl)pyridin-2-yl)amino)pyr azine-2-carbonitrile (CCT245737) J Med Chem. 2016;59:5221–37. doi: 10.1021/acs.jmedchem.5b01938. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

