Statins to prevent early cardiac dysfunction in cancer patients at increased cardiotoxicity risk receiving anthracyclines
- PMID: 37120736
- PMCID: PMC10509566
- DOI: 10.1093/ehjcvp/pvad031
Statins to prevent early cardiac dysfunction in cancer patients at increased cardiotoxicity risk receiving anthracyclines
Abstract
Background and aims: Anthracyclines can cause cancer therapy-related cardiac dysfunction (CTRCD). We aimed to assess whether statins prevent decline in left ventricular ejection fraction (LVEF) in anthracycline-treated patients at increased risk for CTRCD.
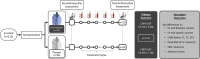
Methods: In this multicenter double-blinded, placebo-controlled trial, patients with cancer at increased risk of anthracycline-related CTRCD (per ASCO guidelines) were randomly assigned to atorvastatin 40 mg or placebo once-daily. Cardiovascular magnetic resonance (CMR) imaging was performed before and within 4 weeks after anthracyclines. Blood biomarkers were measured at every cycle. The primary outcome was post-anthracycline LVEF, adjusted for baseline. CTRCD was defined as a fall in LVEF by >10% to <53%. Secondary endpoints included left ventricular (LV) volumes, CTRCD, CMR tissue characterization, high sensitivity troponin I (hsTnI), and B-type natriuretic peptide (BNP).
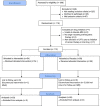
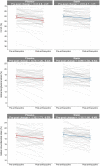
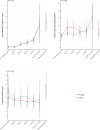
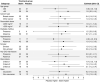
Results: We randomized 112 patients (56.9 ± 13.6 years, 87 female, and 73 with breast cancer): 54 to atorvastatin and 58 to placebo. Post-anthracycline CMR was performed 22 (13-27) days from last anthracycline dose. Post-anthracycline LVEF did not differ between the atorvastatin and placebo groups (57.3 ± 5.8% and 55.9 ± 7.4%, respectively) when adjusted for baseline LVEF (P = 0.34). There were no significant between-group differences in post-anthracycline LV end-diastolic (P = 0.20) or end-systolic volume (P = 0.12), CMR myocardial edema and/or fibrosis (P = 0.06-0.47), or peak hsTnI (P ≥ 0.99) and BNP (P = 0.23). CTRCD incidence was similar (4% versus 4%, P ≥ 0.99). There was no difference in adverse events.
Conclusions: In patients at increased risk of CTRCD, primary prevention with atorvastatin during anthracycline therapy did not ameliorate early LVEF decline, LV remodeling, CTRCD, change in serum cardiac biomarkers, or CMR myocardial tissue changes.
Trial registration: NCT03186404.
Keywords: Anthracycline; Cardiotoxicity; Magnetic resonance imaging; Primary prevention; Statins.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures
References
-
- Demissei BG, Fan Y, Qian Y, Cheng HG, Smith AM, Shimamoto K, Vedage N, Narayan HK, Scherrer-Crosbie M, Davatzikos C, Ky B. Left ventricular segmental strain and the prediction of cancer therapy-related cardiac dysfunction. Eur Heart J Cardiovasc Imaging 2021;22:418–426. 10.1093/ehjci/jeaa288 - DOI - PMC - PubMed
-
- Suerken CK, D'Agostino RB Jr., Jordan JH, Melendez GC, Vasu S, Lamar ZS, Hundley WG. Simultaneous Left Ventricular Volume and Strain Changes During Chemotherapy Associate With 2-Year Postchemotherapy Measures of Left Ventricular Ejection Fraction. J Am Heart Assoc 2020;9:e015400. 10.1161/JAHA.119.015400 - DOI - PMC - PubMed
-
- Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981–1988. 10.1161/CIRCULATIONAHA.114.013777 - DOI - PubMed
-
- Negishi T, Thavendiranathan P, Penicka M, Lemieux J, Murbraech K, Miyazaki S, Shirazi M, Santoro C, Cho G-Y, Popescu BA, Kosmala W, Costello B, la Gerche A, Mottram P, Thomas L, Seldrum S, Hristova K, Bansal M, Kurosawa K, Fukuda N, Yamada H, Izumo M, Tajiri K, Sinski M, Vinereanu D, Shkolnik E, Banchs J, Kutty S, Negishi K, Marwick TH. Cardioprotection Using Strain-Guided Management of Potentially Cardiotoxic Cancer Therapy: 3-Year Results of the SUCCOUR Trial. JACC: Cardiovascular Imaging 2023;16:269–278. 10.1016/j.jcmg.2022.10.010 - DOI - PubMed

