IL-8 concurrently promotes idiopathic pulmonary fibrosis mesenchymal progenitor cell senescence and PD-L1 expression enabling escape from immune cell surveillance
- PMID: 37121574
- PMCID: PMC10228676
- DOI: 10.1152/ajplung.00028.2023
IL-8 concurrently promotes idiopathic pulmonary fibrosis mesenchymal progenitor cell senescence and PD-L1 expression enabling escape from immune cell surveillance
Abstract
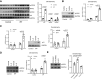
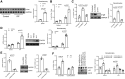
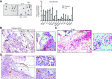
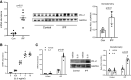
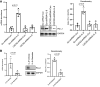
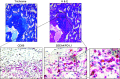
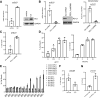
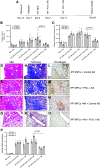
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrotic lung disease. We discovered fibrogenic mesenchymal progenitor cells (MPCs) in the lungs of IPF patients that display cell-autonomous fibrogenicity and drive fibrotic progression. In a study of the IPF MPC nuclear proteome, we identified DNA damage as one of the most altered functions in IPF MPCs. In prior work we found that IL-8 drives IPF MPC self-renewal. IL-8 can promote replicative stress and DNA damage and induce senescence through the CXCR2 receptor. We hypothesized that IL-8 promotes DNA damage-mediated senescence in IPF MPCs. We show that IL-8 induces DNA damage and promotes IPF MPC senescence. We discovered that IL-8 concurrently promotes senescence and upregulation of the programmed death ligand 1 (PD-L1) in a CXCR2-dependent manner. Disruption of programmed cell death protein-1 (PD-1)-PD-L1 interaction promotes natural killer (NK) cell killing of IPF MPCs in vitro and arrests IPF MPC-mediated experimental lung fibrosis in vivo. Immunohistochemical (IHC) analysis of IPF lung tissue identified PD-L1-expressing IPF MPCs codistributing with NK cells and β-galactosidase-positive cells. Our data indicate that IL-8 simultaneously promotes IPF MPC DNA damage-induced senescence and high PD-L1 expression, enabling IPF MPCs to elude immune cell-targeted removal. Disruption of PD-1-PD-L1 interaction may limit IPF MPC-mediated fibrotic progression.NEW & NOTEWORTHY Here we show that IL-8 concurrently promotes senescence and upregulation of PD-L1 in IPF MPCs. IHC analysis identifies the presence of senescent IPF MPCs intermingled with NK cells in the fibroblastic focus, suggesting that senescent MPCs elude immune cell surveillance. We demonstrate that disruption of PD-1/PD-L1 interaction promotes NK cell killing of IPF MPCs and arrests IPF MPC-mediated experimental lung fibrosis. Disruption of PD-1/PD-L1 interaction may be one means to limit fibrotic progression.
Keywords: PD-L1; idiopathic pulmonary fibrosis; mesenchymal progenitor cells; senescence.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

Similar articles
-
IL-8 mediates idiopathic pulmonary fibrosis mesenchymal progenitor cell fibrogenicity.Am J Physiol Lung Cell Mol Physiol. 2018 Jan 1;314(1):L127-L136. doi: 10.1152/ajplung.00200.2017. Epub 2017 Aug 31. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 28860143 Free PMC article.
-
A CD44/Brg1 nuclear complex confers mesenchymal progenitor cells with enhanced fibrogenicity in idiopathic pulmonary fibrosis.JCI Insight. 2021 May 10;6(9):e144652. doi: 10.1172/jci.insight.144652. JCI Insight. 2021. PMID: 33822772 Free PMC article.
-
Hyaluronan/CD44 axis regulates S100A4-mediated mesenchymal progenitor cell fibrogenicity in idiopathic pulmonary fibrosis.Am J Physiol Lung Cell Mol Physiol. 2021 May 1;320(5):L926-L941. doi: 10.1152/ajplung.00456.2020. Epub 2021 Mar 10. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 33719561 Free PMC article.
-
The role of PD-1/PD-L1 axis in idiopathic pulmonary fibrosis: Friend or foe?Front Immunol. 2022 Dec 5;13:1022228. doi: 10.3389/fimmu.2022.1022228. eCollection 2022. Front Immunol. 2022. PMID: 36544757 Free PMC article. Review.
-
The role of the immunosuppressive PD-1/PD-L1 checkpoint pathway in the aging process and age-related diseases.J Mol Med (Berl). 2024 Jun;102(6):733-750. doi: 10.1007/s00109-024-02444-6. Epub 2024 Apr 11. J Mol Med (Berl). 2024. PMID: 38600305 Free PMC article. Review.
Cited by
-
The Chemokine System as a Key Regulator of Pulmonary Fibrosis: Converging Pathways in Human Idiopathic Pulmonary Fibrosis (IPF) and the Bleomycin-Induced Lung Fibrosis Model in Mice.Cells. 2024 Dec 12;13(24):2058. doi: 10.3390/cells13242058. Cells. 2024. PMID: 39768150 Free PMC article. Review.
-
Antioxidant Senotherapy by Natural Compounds: A Beneficial Partner in Cancer Treatment.Antioxidants (Basel). 2025 Feb 10;14(2):199. doi: 10.3390/antiox14020199. Antioxidants (Basel). 2025. PMID: 40002385 Free PMC article. Review.
-
Expression of PD-1/PD-L1 axis in mediastinal lymph nodes and lung tissue of human and experimental lung fibrosis indicates a potential therapeutic target for idiopathic pulmonary fibrosis.Respir Res. 2023 Nov 14;24(1):279. doi: 10.1186/s12931-023-02551-x. Respir Res. 2023. PMID: 37964265 Free PMC article.
-
Advances in understanding the role of interleukins in pulmonary fibrosis (Review).Exp Ther Med. 2024 Nov 28;29(2):25. doi: 10.3892/etm.2024.12775. eCollection 2025 Feb. Exp Ther Med. 2024. PMID: 39650776 Free PMC article. Review.
-
Interstitial lung diseases with concomitant lung cancer: a data mining approach revealing a complex condition with gender- and immune-associated specific implications.Front Oncol. 2024 Dec 17;14:1488157. doi: 10.3389/fonc.2024.1488157. eCollection 2024. Front Oncol. 2024. PMID: 39741973 Free PMC article.
References
-
- Xia H, Bodempudi V, Benyumov A, Hergert P, Tank D, Herrera J, Braziunas J, Larsson O, Parker M, Rossi D, Smith K, Peterson M, Limper A, Jessurun J, Connett J, Ingbar D, Phan S, Bitterman PB, Henke CA. Identification of a cell-of-origin for fibroblasts comprising the fibrotic reticulum in idiopathic pulmonary fibrosis. Am J Pathol 184: 1369–1383, 2014. doi:10.1016/j.ajpath.2014.01.012. - DOI - PMC - PubMed
-
- Xia H, Gilbertsen A, Herrera J, Racila E, Smith K, Peterson M, Griffin T, Benyumov A, Yang L, Bitterman PB, Henke CA. Calcium-binding protein S100A4 confers mesenchymal progenitor cell fibrogenicity in idiopathic pulmonary fibrosis. J Clin Invest 127: 2586–2597, 2017. doi:10.1172/JCI90832. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

