Glia maturation factor-γ is required for initiation and maintenance of hematopoietic stem and progenitor cells
- PMID: 37122014
- PMCID: PMC10150485
- DOI: 10.1186/s13287-023-03328-1
Glia maturation factor-γ is required for initiation and maintenance of hematopoietic stem and progenitor cells
Abstract
Background: In vertebrates, hematopoietic stem and progenitor cells (HSPCs) emerge from hemogenic endothelium in the floor of the dorsal aorta and subsequently migrate to secondary niches where they expand and differentiate into committed lineages. Glia maturation factor γ (gmfg) is a key regulator of actin dynamics that was shown to be highly expressed in hematopoietic tissue. Our goal is to investigate the role and mechanism of gmfg in embryonic HSPC development.
Methods: In-depth bioinformatics analysis of our published RNA-seq data identified gmfg as a cogent candidate gene implicated in HSPC development. Loss and gain-of-function strategies were applied to study the biological function of gmfg. Whole-mount in situ hybridization, confocal microscopy, flow cytometry, and western blotting were used to evaluate changes in the number of various hematopoietic cells and expression levels of cell proliferation, cell apoptosis and hematopoietic-related markers. RNA-seq was performed to screen signaling pathways responsible for gmfg deficiency-induced defects in HSPC initiation. The effect of gmfg on YAP sublocalization was assessed in vitro by utilizing HUVEC cell line.
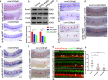
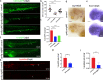
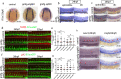
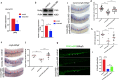
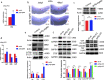
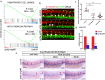
Results: We took advantage of zebrafish embryos to illustrate that loss of gmfg impaired HSPC initiation and maintenance. In gmfg-deficient embryos, the number of hemogenic endothelium and HSPCs was significantly reduced, with the accompanying decreased number of erythrocytes, myelocytes and lymphocytes. We found that blood flow modulates gmfg expression and gmfg overexpression could partially rescue the reduction of HSPCs in the absence of blood flow. Assays in zebrafish and HUVEC showed that gmfg deficiency suppressed the activity of YAP, a well-established blood flow mediator, by preventing its shuttling from cytoplasm to nucleus. During HSPC initiation, loss of gmfg resulted in Notch inactivation and the induction of Notch intracellular domain could partially restore the HSPC loss in gmfg-deficient embryos.
Conclusions: We conclude that gmfg mediates blood flow-induced HSPC maintenance via regulation of YAP, and contributes to HSPC initiation through the modulation of Notch signaling. Our findings reveal a brand-new aspect of gmfg function and highlight a novel mechanism for embryonic HSPC development.
Keywords: Blood flow; Glia maturation factor-γ; HSPC; Notch; Yap; Zebrafish.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The expression of glia maturation factors and the effect of glia maturation factor-γ on angiogenic sprouting in zebrafish.Exp Cell Res. 2013 Mar 10;319(5):707-17. doi: 10.1016/j.yexcr.2013.01.004. Epub 2013 Jan 16. Exp Cell Res. 2013. PMID: 23333559
-
DNA methylation safeguards the generation of hematopoietic stem and progenitor cells by repression of Notch signaling.Development. 2022 May 15;149(10):dev200390. doi: 10.1242/dev.200390. Epub 2022 May 25. Development. 2022. PMID: 35502759 Free PMC article.
-
Glia maturation factor-gamma is preferentially expressed in microvascular endothelial and inflammatory cells and modulates actin cytoskeleton reorganization.Circ Res. 2006 Aug 18;99(4):424-33. doi: 10.1161/01.RES.0000237662.23539.0b. Epub 2006 Jul 27. Circ Res. 2006. PMID: 16873721
-
Sequential regulation of hemogenic fate and hematopoietic stem and progenitor cell formation from arterial endothelium by Ezh1/2.Stem Cell Reports. 2021 Jul 13;16(7):1718-1734. doi: 10.1016/j.stemcr.2021.05.014. Epub 2021 Jun 17. Stem Cell Reports. 2021. PMID: 34143974 Free PMC article.
-
[Review of the research of glia maturation factor and cloning of human and rat glia maturation factor-gamma (GMFG) cDNA].Nihon Shinkei Seishin Yakurigaku Zasshi. 2001 Feb;21(1):15-20. Nihon Shinkei Seishin Yakurigaku Zasshi. 2001. PMID: 11400321 Review. Japanese.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

