French guidelines for the etiological workup of eosinophilia and the management of hypereosinophilic syndromes
- PMID: 37122022
- PMCID: PMC10148979
- DOI: 10.1186/s13023-023-02696-4
French guidelines for the etiological workup of eosinophilia and the management of hypereosinophilic syndromes
Abstract
Eosinophilic-related clinical manifestations are protean and the underlying conditions underpinning eosinophilia are highly diverse. The etiological workup of unexplained eosinophilia/hypereosinophilia can be challenging, and can lead sometimes to extensive, inappropriate, costly and/or invasive investigations. To date, guidelines for the etiological workup and management of eosinophilia are mainly issued by hematologists, and thus mostly cover the scope of clonal hypereosinophilic syndromes (HES). Here, thanks to an extensive literature review, and thanks to the joint work of a large panel of experts involving physicians from both adult and pediatric medicine and from various subspecialties (as well as a representative of a patients' association representative), we provide recommendations for both the step-by step diagnostic workup of eosinophilia (whether unexplained or within specific contexts) as well as the management and follow-up of the full spectrum of eosinophilic disorders (including clonal, reactive, lymphocytic and idiopathic HES, as well as single-organ diseases). Didactic prescription summaries intended to facilitate the prescription of eosinophil-targeted drugs are also provided, as are practical diagnostic and therapeutic algorithms. Lastly, this set of recommendations also includes a summary intended for general practitioners, as well as an overview of the therapeutic patient education program set up by the French reference center for HES. Further updates will be mandatory as new validated information emerges.
Keywords: Hypereosinophilia; Hypereosinophilic syndromes; Management; Recommendation.
© 2023. The Author(s).
Conflict of interest statement
MG reports consulting fees from GlaxoSmithKline and AstraZeneca; speaking fees from GlaxoSmithKline, AstraZeneca and Sanofi; JRoh reports consulting fees from GlaxoSmithKline; speaking fees from GlaxoSmithKline; VC reports consulting fees from CSL Behring and AstraZeneca; speaking fees from AstraZeneca; ND reports consulting fees from Bristol Myers Squibb; SF reports consulting fees from Abyonix Pharma, Novartis, GlaxoSmithKline, Sanofi-Genzyme, CSL Vifor, Alexion; speeking fees from Baxter, Sanofi-Genzyme, CSL-Vifor; FG reports consulting fees from Nestlé; MH reports consulting fees from GlaxoSmithKline and Blueprint; speaking fees from Novartis, GlaxoSmithKline and Sanofi. AM reports consulting fees form GlaxoSmithKline, AstraZeneca and Novartis; speeking fees from GlaxoSmithKline, AstraZeneca and Novartis; TM reports consulting fees from Amgen, Chugai, Boehringer; speaking fees from GlaxoSmithKline; JRos reports speeking fees from Novartis; JSD reports consulting fees from Novartis; DSS reports grants from AstraZeneca and Sanofi-Genzyme; consulting fees from Astra-Zeneca, Novartis, Sanofi; speeking fees from Novartis and Sanofi-Genzyme; JFV reports consulting fees from GlaxoSmithKline; GL reports consulting fees from GlaxoSmithKline and AstraZeneca; speaking fees from GlaxoSmithKline and AstraZeneca. JEK reports consulting fees from GlaxoSmithKline and AstraZeneca.
Figures
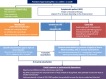

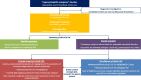
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

