Big tau aggregation disrupts microtubule tyrosination and causes myocardial diastolic dysfunction: from discovery to therapy
- PMID: 37122097
- PMCID: PMC10324644
- DOI: 10.1093/eurheartj/ehad205
Big tau aggregation disrupts microtubule tyrosination and causes myocardial diastolic dysfunction: from discovery to therapy
Erratum in
-
Erratum to: Big tau aggregation disrupts microtubule tyrosination and causes myocardial diastolic dysfunction: from discovery to therapy.Eur Heart J. 2023 Sep 14;44(35):3335. doi: 10.1093/eurheartj/ehad439. Eur Heart J. 2023. PMID: 37656892 Free PMC article. No abstract available.
Abstract
Background: Amyloid plaques and neurofibrillary tangles, the molecular lesions that characterize Alzheimer's disease (AD) and other forms of dementia, are emerging as determinants of proteinopathies 'beyond the brain'. This study aims to establish tau's putative pathophysiological mechanistic roles and potential future therapeutic targeting of tau in heart failure (HF).
Methods and results: A mouse model of tauopathy and human myocardial and brain tissue from patients with HF, AD, and controls was employed in this study. Tau protein expression was examined together with its distribution, and in vitro tau-related pathophysiological mechanisms were identified using a variety of biochemical, imaging, and functional approaches. A novel tau-targeting immunotherapy was tested to explore tau-targeted therapeutic potential in HF. Tau is expressed in normal and diseased human hearts, in contradistinction to the current oft-cited observation that tau is expressed specifically in the brain. Notably, the main cardiac isoform is high-molecular-weight (HMW) tau (also known as big tau), and hyperphosphorylated tau segregates in aggregates in HF and AD hearts. As previously described for amyloid-beta, the tauopathy phenotype in human myocardium is of diastolic dysfunction. Perturbation in the tubulin code, specifically a loss of tyrosinated microtubules, emerged as a potential mechanism of myocardial tauopathy. Monoclonal anti-tau antibody therapy improved myocardial function and clearance of toxic aggregates in mice, supporting tau as a potential target for novel HF immunotherapy.
Conclusion: The study presents new mechanistic evidence and potential treatment for the brain-heart tauopathy axis in myocardial and brain degenerative diseases and ageing.
Keywords: Alzheimer’s; HMW tau; Heart failure; immunotherapy; tau protein.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest All authors declare no conflict of interest for this contribution.
Figures


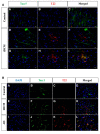

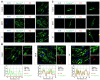
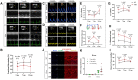

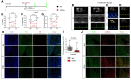
Comment in
-
Aggregation of big tau disrupts microtubules and causes diastolic dysfunction.Nat Rev Cardiol. 2023 Jul;20(7):441. doi: 10.1038/s41569-023-00890-2. Nat Rev Cardiol. 2023. PMID: 37198343 No abstract available.
References
-
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 2004;5:347–360. - PubMed
-
- Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, et al. . Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam study. Ann Neurol 2005;57:789–794. - PubMed
-
- Stamatelopoulos K, Sibbing D, Rallidis LS, Georgiopoulos G, Stakos D, Braun S, et al. . Amyloid-beta (1–40) and the risk of death from cardiovascular causes in patients with coronary heart disease. J Am Coll Cardiol 2015;65:904–916. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

