Kinase-Modulated Bioluminescent Indicators Enable Noninvasive Imaging of Drug Activity in the Brain
- PMID: 37122464
- PMCID: PMC10141594
- DOI: 10.1021/acscentsci.3c00074
Kinase-Modulated Bioluminescent Indicators Enable Noninvasive Imaging of Drug Activity in the Brain
Abstract
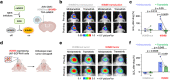
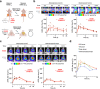
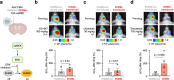
Aberrant kinase activity contributes to the pathogenesis of brain cancers, neurodegeneration, and neuropsychiatric diseases, but identifying kinase inhibitors that function in the brain is challenging. Drug levels in blood do not predict efficacy in the brain because the blood-brain barrier prevents entry of most compounds. Rather, assessing kinase inhibition in the brain requires tissue dissection and biochemical analysis, a time-consuming and resource-intensive process. Here, we report kinase-modulated bioluminescent indicators (KiMBIs) for noninvasive longitudinal imaging of drug activity in the brain based on a recently optimized luciferase-luciferin system. We develop an ERK KiMBI to report inhibitors of the Ras-Raf-MEK-ERK pathway, for which no bioluminescent indicators previously existed. ERK KiMBI discriminates between brain-penetrant and nonpenetrant MEK inhibitors, reveals blood-tumor barrier leakiness in xenograft models, and reports MEK inhibitor pharmacodynamics in native brain tissues and intracranial xenografts. Finally, we use ERK KiMBI to screen ERK inhibitors for brain efficacy, identifying temuterkib as a promising brain-active ERK inhibitor, a result not predicted from chemical characteristics alone. Thus, KiMBIs enable the rapid identification and pharmacodynamic characterization of kinase inhibitors suitable for treating brain diseases.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): Y.S., Y.W., and M.Z.L. have applied for a patent relating to the content of the manuscript.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

