The reporting quality and transparency of orthopaedic studies using Bayesian analysis requires improvement: A systematic review
- PMID: 37122488
- PMCID: PMC10130591
- DOI: 10.1016/j.conctc.2023.101132
The reporting quality and transparency of orthopaedic studies using Bayesian analysis requires improvement: A systematic review
Abstract
Background: Bayesian methods are being used more frequently in orthopaedics. To advance the use and transparent reporting of Bayesian studies, reporting guidelines have been recommended. There is currently little known about the use or applications of Bayesian analysis in orthopedics including adherence to recommended reporting guidelines. The objective is to investigate the reporting of Bayesian analysis in orthopedic surgery studies; specifically, to evaluate if these papers adhere to reporting guidelines.
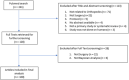
Methods: We searched PUBMED to December 2nd, 2020. Two reviewers independently identified studies and full-text screening. We included studies that focused on one or more orthopaedic surgical interventions and used Bayesian methods.
Results: After full-text review, 100 articles were included. The most frequent study designs were meta-analysis or network meta-analysis (56%, 95% CI 46-65) and cohort studies (25%, 95% CI 18-34). Joint replacement was the most common subspecialty (33%, 95% CI 25-43). We found that studies infrequently reported key concepts in Bayesian analysis including, specifying the prior distribution (37-39%), justifying the prior distribution (18%), the sensitivity to different priors (7-8%), and the statistical model used (22%). In contrast, general methodological items on the checklists were largely well reported.
Conclusions: There is an opportunity to improve reporting quality and transparency of orthopaedic studies using Bayesian analysis by encouraging adherence to reporting guidelines such as ROBUST, JASP, and BayesWatch. There is an opportunity to better report prior distributions, sensitivity analyses, and the statistical models used.
Keywords: Bayesian analysis; Orthopaedic surgery; Systematic review.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
-
- van de Schoot R., Winters S.D., Ryan O., Zondervan-Zwijnenburg M., Depaoli S. A systematic review of Bayesian articles in psychology: the last 25 years. Psychol. Methods. 2017;22:2 217–239. - PubMed
-
- Kruschke J.K., Liddell T.M. Bayesian data analysis for newcomers. Psychon. Bull. Rev. 2018;25:155–177. - PubMed
-
- Rietbergen C., Debray T.P.A., Klugkist I., Janssen K.J.M., Moons K.G.M. Reporting of Bayesian analysis in epidemiologic research should become more transparent. J. Clin. Epidemiol. 2017;86:51–58 e2. - PubMed
-
- Ferreira D., Vivot A., Diemunsch P., Meyer N. Bayesian analysis from phase III trials was underused and poorly reported: a systematic review. J. Clin. Epidemiol. 2020;123:107–113. - PubMed
-
- Zhai J., Hongbo C., Ren M., Mu W., Lv S., Si J., Wang H., Chen J., Shang H. Reporting of core items in hierarchical Bayesian analysis for aggregating N-of-1 trials to estimate population treatment effects is suboptimal. J. Clin. Epidemiol. 2016;76:99–107. doi: 10.1016/j.jclinepi.2016.02.023. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources