Effectiveness of Helicobacter pylori eradication in the treatment of early-stage gastric mucosa-associated lymphoid tissue lymphoma: An up-to-date meta-analysis
- PMID: 37122607
- PMCID: PMC10130965
- DOI: 10.3748/wjg.v29.i14.2202
Effectiveness of Helicobacter pylori eradication in the treatment of early-stage gastric mucosa-associated lymphoid tissue lymphoma: An up-to-date meta-analysis
Abstract
Background: Gastric mucosa-associated lymphoid tissue (MALT) lymphoma (GML) is usually a low-grade B-cell neoplasia strongly associated with Helicobacter pylori (H. pylori)-induced chronic gastritis. Clinical practice guidelines currently recommend H. pylori eradication as the preferred initial treatment for early-stage GML. To determine the practical effect of bacterial eradication as the sole initial therapy for early-stage GML, an updated analysis and review of available evidence is imperative.
Aim: To perform a meta-analysis to assess the rate of complete remission (CR) of H. pylori-positive early-stage GML following bacterial eradication.
Methods: We performed independent, computer-assisted literature searches using the PubMed/MEDLINE, Embase, and Cochrane Central databases through September 2022. Prospective and retrospective observational studies evaluating the CR of early-stage GML following bacterial eradication in H. pylori-positive patients. The risk of bias was assessed using Joanna Briggs Institute (JBI) Critical Appraisal Tools. The pooled estimate of the complete histopathological remission rate and respective confidence intervals (95%CI) were calculated following the random-effects model. Heterogeneity and inconsistency were assessed using Cochran's Q test and I2 statistic, and heterogeneity was defined as P < 0.01 and I² > 50%, respectively. Subgroup and meta-regression analyses were conducted to explore potential sources of heterogeneity.
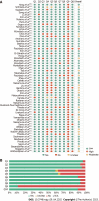
Results: The titles and abstracts of 1576 studies were screened; 96 articles were retrieved and selected for full-text reading. Finally, 61 studies were included in the proportional meta-analysis (P-MA). Forty-six were prospective and fifteen were retrospective uncontrolled, single-arm, observational studies. The overall risk of bias was low to moderate in all but a single report, with an average critical appraisal score across all studies of 79.02%. A total of 2936 H. pylori-positive early-stage GML patients, in whom H. pylori was successfully eradicated, were included in the analysis. The pooled CR of H. pylori-positive early-stage GML after bacterial eradication was 75.18% (95%CI: 70.45%-79.91%). P-MA indicated the substantial heterogeneity in CR reported across studies (I 2 = 92%; P < 0.01). Meta-regression analysis identified statistically significant effect modifiers, including the proportion of patients with t(11;18)(q21;q21)-positive GML and the risk of bias in each study.
Conclusion: Comprehensive synthesis of available evidence suggests that H. pylori eradication is effective as the sole initial therapy for early-stage GML. Although the substantial heterogeneity observed across studies limits the interpretation of the pooled overall CR, the present study is a relevant to informing clinical practice.
Keywords: B-cell; Eradication therapy; Gastric mucosa-associated lymphoid tissue lymphoma; Helicobacter pylori; Lymphoma; Marginal zone; Stomach lymphoma; Therapeutics.
©The Author(s) 2023. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures


References
-
- Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D, Calaminici M, Chadburn A, Chan JKC, Cheuk W, Chng WJ, Choi JK, Chuang SS, Coupland SE, Czader M, Dave SS, de Jong D, Du MQ, Elenitoba-Johnson KS, Ferry J, Geyer J, Gratzinger D, Guitart J, Gujral S, Harris M, Harrison CJ, Hartmann S, Hochhaus A, Jansen PM, Karube K, Kempf W, Khoury J, Kimura H, Klapper W, Kovach AE, Kumar S, Lazar AJ, Lazzi S, Leoncini L, Leung N, Leventaki V, Li XQ, Lim MS, Liu WP, Louissaint A Jr, Marcogliese A, Medeiros LJ, Michal M, Miranda RN, Mitteldorf C, Montes-Moreno S, Morice W, Nardi V, Naresh KN, Natkunam Y, Ng SB, Oschlies I, Ott G, Parrens M, Pulitzer M, Rajkumar SV, Rawstron AC, Rech K, Rosenwald A, Said J, Sarkozy C, Sayed S, Saygin C, Schuh A, Sewell W, Siebert R, Sohani AR, Tooze R, Traverse-Glehen A, Vega F, Vergier B, Wechalekar AD, Wood B, Xerri L, Xiao W. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022;36:1720–1748. - PMC - PubMed
-
- Rossi D, Bertoni F, Zucca E. Marginal-Zone Lymphomas. N Engl J Med. 2022;386:568–581. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

