Salmonella enterica serovar Paratyphi A-induced immune response in Caenorhabditis elegans depends on MAPK pathways and DAF-16
- PMID: 37122724
- PMCID: PMC10132459
- DOI: 10.3389/fimmu.2023.1118003
Salmonella enterica serovar Paratyphi A-induced immune response in Caenorhabditis elegans depends on MAPK pathways and DAF-16
Abstract
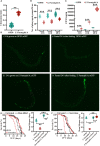
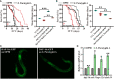
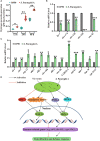
Salmonella enterica serovar Paratyphi A (S. Paratyphi A) is a pathogen that can cause enteric fever. According to the recent epidemic trends of typhoid fever, S. Paratyphi A has been the major important causative factor in paratyphoid fever. An effective vaccine for S. Paratyphi A has not been developed, which made it a tricky public health concern. Until now, how S. Paratyphi A interacts with organisms remain unknown. Here using lifespan assay, we found that S. Paratyphi A could infect Caenorhabditis elegans (C. elegans) at 25°C, and attenuate thermotolerance. The immune response of C. elegans was mediated by tir-1, nsy-1, sek-1, pmk-1, mpk-1, skn-1, daf-2 and daf-16, suggesting that S. Paratyphi A could regulate the MAPK and insulin pathways. Furthermore, we observed several phenotypical changes when C. elegans were fed S. Paratyphi A, including an accelerated decline in body movement, reduced the reproductive capacity, shortened spawning cycle, strong preference for OP50, arrested pharyngeal pumping and colonization of the intestinal lumen. The virulence of S. Paratyphi A requires living bacteria and is not mediated by secreting toxin. Using hydrogen peroxide analysis and quantitative RT-PCR, we discovered that S. Paratyphi A could increase oxidative stress and regulate the immune response in C. elegans. Our results sheds light on the infection mechanisms of S. Paratyphi A and lays a foundation for drugs and vaccine development.
Keywords: Caenorhabditis elegans; MAPK; Salmonella enterica serovar Paratyphi A; immune; oxidative stress.
Copyright © 2023 Ding, Zhang, Tao, Chen, Liu, Dong, Ma, Pan, He and Zeng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

