Transition-metal-catalyzed C-H bond activation as a sustainable strategy for the synthesis of fluorinated molecules: an overview
- PMID: 37123090
- PMCID: PMC10130906
- DOI: 10.3762/bjoc.19.35
Transition-metal-catalyzed C-H bond activation as a sustainable strategy for the synthesis of fluorinated molecules: an overview
Abstract
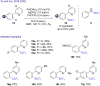
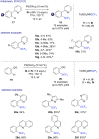
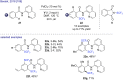
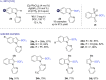
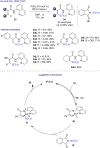
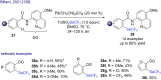
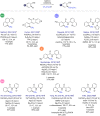
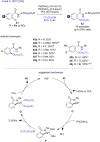
The last decade has witnessed the emergence of innovative synthetic tools for the synthesis of fluorinated molecules. Among these approaches, the transition-metal-catalyzed functionalization of various scaffolds with a panel of fluorinated groups (XRF, X = S, Se, O) offered straightforward access to high value-added compounds. This review will highlight the main advances made in the field with the transition-metal-catalyzed functionalization of C(sp2) and C(sp3) centers with SCF3, SeCF3, or OCH2CF3 groups among others, by C-H bond activation. The scope and limitations of these transformations are discussed in this review.
Keywords: C–H bond activation; emergent fluorinated groups; homogeneous catalysis; organofluorine chemistry; palladium; synthetic method.
Copyright © 2023, Monsigny et al.
Figures


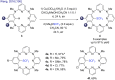
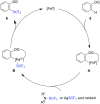
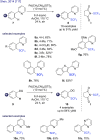
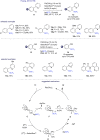
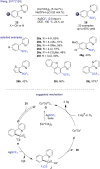
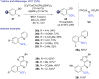
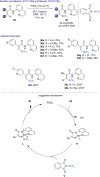
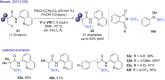


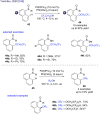
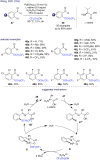
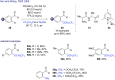
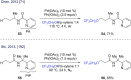
References
-
- Smart B E. J Fluorine Chem. 2001;109:3–11. doi: 10.1016/s0022-1139(01)00375-x. - DOI
Publication types
LinkOut - more resources
Full Text Sources
