Oxidative Degradation of Sequence-Defined Peptoid Oligomers
- PMID: 37123435
- PMCID: PMC10147340
- DOI: 10.1039/d2me00179a
Oxidative Degradation of Sequence-Defined Peptoid Oligomers
Abstract
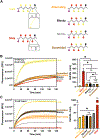
Due to their N-substitution, peptoids are generally regarded as resistant to biological degradation, such as enzymatic and hydrolytic mechanisms. This stability is an especially attractive feature for therapeutic development and is a selling point of many previous biological studies. However, another key mode of degradation remains to be fully explored, namely oxidative degradation mediated by reactive oxygen and nitrogen species (ROS/RNS). ROS and RNS are biologically relevant in numerous contexts where biomaterials may be present, thus, improving understanding of peptoid oxidative susceptibility is crucial to exploit their full potential in the biomaterials field, where an oxidatively-labile but enzymatically stable molecule can offer attractive properties. Toward this end, we demonstrate a fundamental characterization of sequence-defined peptoid chains in the presence of chemically generated ROS, as compared to ROS-susceptible peptides such as proline and lysine oligomers. Lysine oligomers showed the fastest degradation rates to ROS and the enzyme trypsin. Peptoids degraded in metal catalyzed oxidation conditions at rates on par with poly(prolines), while maintaining resistance to enzymatic degradation. Furthermore, lysine-containing peptide-peptoid hybrid molecules showed tunability in both ROS-mediated and enzyme-mediated degradation, with rates intermediate to lysine and peptoid oligomers. When lysine-mimetic side-chains were incorporated into a peptoid backbone, the rate of degradation matched that of the lysine peptide oligomers, but remained resistant to enzymatic degradation. These results expand understanding of peptoid degradation to oxidative and enzymatic mechanisms, and demonstrate the potential for peptoid incorporation into materials where selectivity towards oxidative degradation is necessary, or directed enzymatic susceptibility is desired.
Keywords: Degradation; Peptoids; Reactive Oxygen Species; Sequence-Defined.
Conflict of interest statement
Conflicts of Interest There are no conflicts to declare.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
