Fully resorbable poly-4-hydroxybutyrate (P4HB) mesh for soft tissue repair and reconstruction: A scoping review
- PMID: 37123542
- PMCID: PMC10130450
- DOI: 10.3389/fsurg.2023.1157661
Fully resorbable poly-4-hydroxybutyrate (P4HB) mesh for soft tissue repair and reconstruction: A scoping review
Abstract
Background: Poly-4-hydroxybutyrate (P4HB) is a fully resorbable, biologically-produced polymer with a strength and flexibility comparable to permanent synthetic polymers. The objective was to identify/summarize all peer-reviewed publications involving P4HB mesh.
Methods: A scoping review was conducted within PubMed and included articles published through October 2022.
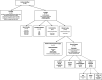
Results: A total of n = 79 studies were identified (n = 12 in vitro/bench; n = 14 preclinical; n = 6 commentaries; n = 50 clinical). Of the clinical studies, n = 40 reported results applicable to hernia and n = 10 to plastic/reconstructive surgery and involved patients of all Centers for Disease Control (CDC) wound classes and Ventral Hernia Working Group (VHWG) grades.
Conclusion: P4HB mesh provides long-term hernia repair strength and exhibits promising clinical outcomes beyond its resorption period. Future studies should include randomized controlled trials comparing P4HB to other biomaterials, as well as optimal patient selection, operative technique, long-term outcomes, minimization of potential mesh-related complications, and potential contraindications/complications for P4HB in hernia/abdominal wall reconstruction.
Keywords: P4HB; Phasix Mesh; hernia recurrence; poly-4-hydroxybutyrate (P4HB); resorbable; surgical site infection (SSI).
© 2023 Deeken, Chen, López-Cano, Martin and Badhwar.
Conflict of interest statement
This project was sponsored by Becton, Dickinson and Company (BD) of Warwick, Rhode Island (USA). CD is the owner of Covalent Bio, LLC, which received consulting fees from BD for this project, as well as other, unrelated projects. DM and AB are employees of BD. DC and ML are consultants for BD. CD reports consulting fees from C.R. Bard, Inc./Davol/Becton Dickinson (BD) during the conduct of the study. DC also reports consulting fees from C.R. Bard, Inc./Davol/Becton Dickinson (BD), Johnson & Johnson, Medtronic, SurgiMatrix, Tissium, Surgical Innovation Associates, Americas Hernia Society Quality Collaborative, Colorado Therapeutics, TelaBio, Osteogenics, Polynovo, MedSkin Solutions, and Aran Biomedical outside the submitted work. In addition, CD is the owner of Covalent Bio, LLC and holds the following issued patents: 2009293001, 2334257, 2,334,257UK, 602009046407.8, 2,334,257FR, 16/043,849 and 2,737,542. DC reports consulting fees from Becton Dickinson (BD) during the conduct of this study. ML reports consulting fees from Becton Dickinson (BD) during the conduct of this study. DM reports employment by Becton Dickinson (BD) during the conduct of this study, as well as outside of the current work. AB reports employment by Becton Dickinson (BD) during the conduct of this study, as well as outside of the current work.
Figures

References
-
- Martin D, Williams S. Medical applications of poly-4-hydroxybutyrate: a strong flexible absorbable biomaterial. Biochem Engr J. (2003) 16:97–105. 10.1089/107632703322066660 - DOI
-
- Shen Y, Zhao Z, Li Y, Liu S, Liu F, Li Z. A facile method to prepare high molecular weight bio-renewable poly(γ-butyrolactone) using a strong base/urea binary synergistic catalytic system. Polym Chem. (2019) 10:1231–7. 10.1039/c8py01812j - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

