Europium-Magnesium-Aluminum-Based Mixed-Metal Oxides as Highly Active Methane Oxychlorination Catalysts
- PMID: 37123594
- PMCID: PMC10127201
- DOI: 10.1021/acscatal.2c06344
Europium-Magnesium-Aluminum-Based Mixed-Metal Oxides as Highly Active Methane Oxychlorination Catalysts
Abstract
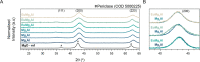
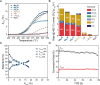
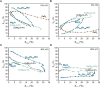
Methane oxychlorination (MOC) is a promising reaction for the production of liquefied methane derivatives. Even though catalyst design is still in its early stages, the general trend is that benchmark catalyst materials have a redox-active site, with, e.g., Cu2+, Ce4+, and Pd2+ as prominent showcase examples. However, with the identification of nonreducible LaOCl moiety as an active center for MOC, it was demonstrated that a redox-active couple is not a requirement to establish a high activity. In this work, we show that Mg2+-Al3+-based mixed-metal oxide (MMO) materials are highly active and stable MOC catalysts. The synergistic interaction between Mg2+ and Al3+ could be exploited due to the fact that a homogeneous distribution of the chemical elements was achieved. This interaction was found to be crucial for the unexpectedly high MOC activity, as reference MgO and γ-Al2O3 materials did not show any significant activity. Operando Raman spectroscopy revealed that Mg2+ acted as a chlorine buffer and subsequently as a chlorinating agent for Al3+, which was the active metal center in the methane activation step. The addition of the redox-active Eu3+ to the nonreducible Mg2+-Al3+ MMO catalyst enabled further tuning of the catalytic performance and made the EuMg3Al MMO catalyst one of the most active MOC catalyst materials reported so far. Combined operando Raman/luminescence spectroscopy revealed that the chlorination behavior of Mg2+ and Eu3+ was correlated, suggesting that Mg2+ also acted as a chlorinating agent for Eu3+. These results indicate that both redox activity and synergistic effects between Eu, Mg, and Al are required to obtain high catalytic performance. The importance of elemental synergy and redox properties is expected to be translatable to the oxychlorination of other hydrocarbons, such as light alkanes, due to large similarities in catalytic chemistry.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Taifan W.; Baltrusaitis J. CH4 Conversion to Value Added Products: Potential, Limitations and Extensions of a Single Step Heterogeneous Catalysis. Appl. Catal., B 2016, 198, 525–547. 10.1016/j.apcatb.2016.05.081. - DOI
-
- Sun L.; Wang Y.; Guan N.; Li L. Methane Activation and Utilization: Current Status and Future Challenges. Energy Technol. 2020, 8, 190082610.1002/ente.201900826. - DOI
LinkOut - more resources
Full Text Sources
