Elucidating the Roles of Nafion/Solvent Formulations in Copper-Catalyzed CO2 Electrolysis
- PMID: 37123601
- PMCID: PMC10127206
- DOI: 10.1021/acscatal.2c05235
Elucidating the Roles of Nafion/Solvent Formulations in Copper-Catalyzed CO2 Electrolysis
Abstract
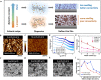
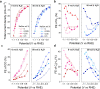
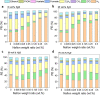
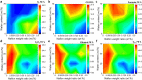
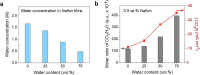
Nafion ionomer, composed of hydrophobic perfluorocarbon backbones and hydrophilic sulfonic acid side chains, is the most widely used additive for preparing catalyst layers (CLs) for electrochemical CO2 reduction, but its impact on the performance of CO2 electrolysis remains poorly understood. Here, we systematically investigate the role of the catalyst ink formulation on CO2 electrolysis using commercial CuO nanoparticles as the model pre-catalyst. We find that the presence of Nafion is essential for achieving stable product distributions due to its ability to stabilize the catalyst morphology under reaction conditions. Moreover, the Nafion content and solvent composition (water/alcohol fraction) regulate the internal structure of Nafion coatings, as well as the catalyst morphology, thereby significantly impacting CO2 electrolysis performance, resulting in variations of C2+ product Faradaic efficiency (FE) by >3×, with C2+ FE ranging from 17 to 54% on carbon paper substrates. Using a combination of ellipsometry and in situ Raman spectroscopy during CO2 reduction, we find that such selectivity differences stem from changes to the local reaction microenvironment. In particular, the combination of high water/alcohol ratios and low Nafion fractions in the catalyst ink results in stable and favorable microenvironments, increasing the local CO2/H2O concentration ratio and promoting high CO surface coverage to facilitate C2+ production in long-term CO2 electrolysis. Therefore, this work provides insights into the critical role of Nafion binders and underlines the importance of optimizing Nafion/solvent formulations as a means of enhancing the performance of electrochemical CO2 reduction systems.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Nitopi S.; Bertheussen E.; Scott S. B.; Liu X.; Engstfeld A. K.; Horch S.; Seger B.; Stephens I. E. L.; Chan K.; Hahn C.; Nørskov J. K.; Jaramillo T. F.; Chorkendorff I. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. 10.1021/acs.chemrev.8b00705. - DOI - PubMed
-
- Han N.; Ding P.; He L.; Li Y.; Li Y. Promises of Main Group Metal–Based Nanostructured Materials for Electrochemical CO2 Reduction to Formate. Adv. Energy Mater. 2020, 10, 190233810.1002/aenm.201902338. - DOI
-
- Zhang X.; Wang Y.; Gu M.; Wang M.; Zhang Z.; Pan W.; Jiang Z.; Zheng H.; Lucero M.; Wang H.; Sterbinsky G. E.; Ma Q.; Wang Y.-G.; Feng Z.; Li J.; Dai H.; Liang Y. Molecular Engineering of Dispersed Nickel Phthalocyanines on Carbon Nanotubes for Selective CO2 Reduction. Nat. Energy 2020, 5, 684–692. 10.1038/s41560-020-0667-9. - DOI
-
- Gong Q.; Ding P.; Xu M.; Zhu X.; Wang M.; Deng J.; Ma Q.; Han N.; Zhu Y.; Lu J.; Feng Z.; Li Y.; Zhou W.; Li Y. Structural Defects on Converted Bismuth Oxide Nanotubes Enable Highly Active Electrocatalysis of Carbon Dioxide Reduction. Nat. Commun. 2019, 10, 280710.1038/s41467-019-10819-4. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
