Vitamin D reduces pain and cartilage destruction in knee osteoarthritis animals through inhibiting the matrix metalloprotease (MMPs) expression
- PMID: 37123896
- PMCID: PMC10130884
- DOI: 10.1016/j.heliyon.2023.e15268
Vitamin D reduces pain and cartilage destruction in knee osteoarthritis animals through inhibiting the matrix metalloprotease (MMPs) expression
Abstract
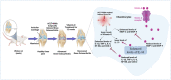
Aim of the study: In this study, we investigated the therapeutic potential of vitamin D (VITD) in OA Wistar rats induced by anterior cruciate ligament transection combined with medial meniscectomy (ACLT + MMx). In ACLT + MMx-induced OA rats, pain severity, cartilage destruction, inflammatory cytokines, and MMPs were all measured.
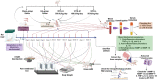
Materials and methods: ACLT + MMx methods were used to induce OA, and pain behavioral studies such as the weight bearing test and paw withdrawal test were performed while the knee width and body weights were also measured. Furthermore, Hematoxylin and Eosin (H&E) staining was used to determine knee histopathological studies, as well as OARSI scoring, cartilage thickness, cartilage width, and cartilage degradation scores. The enzyme-linked immunosorbent assay (ELISA) studies were used to check the serum levels of VITD, C-telopeptide of Type II collagen (CTX-II), and pro-inflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), and anti-inflammatory cytokines interleukin-10 (IL-10), and MMPs (MMP-3, MMP-9, and MMP-13). Finally, the reverse transcription polymerase chain reaction (RT-PCR) test was used to determine the levels of MMPs, nuclear factor-kappa B (NF-κB), TNF-α, IL-6, and IL-10 in IL-1β stimulated chondrocytes.
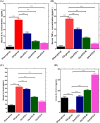
Results: The oral VITD supplement significantly reduced OA pain, inflammation, cartilage destruction, and MMPs levels. Furthermore, serum VITD levels increased while CTX-II levels decreased, indicating that VITD reduced cartilage degradation effectively. Moreover, VITD supplementation reduced the expression of pro-inflammatory TNF-α, IL-1β, and IL-6 cytokines while increasing the expression of anti-inflammatory IL-10. The elevation of MMPs after ACLT + MMx surgery contributed to articular cartilage destruction, which was reduced by VITD supplementation. Finally, VITD supplementation significantly reduces serum levels of MMPs, IL-1β, TNF-α, and IL-6 while increasing IL-10 levels. Then, using the in-vitro cytotoxicity (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) MTT assay, examine the cytotoxicity profile of VITD in rat chondrocytes after stimulated with IL-1β, which shows no toxicity in the dose range of VITD 0-500 IU. Finally, RT-PCR studies in IL-1β stimulated rat chondrocytes revealed that VITD (50, 100, and 500 IU) significantly reduced the mRNA levels of MMPs, NF-κB, TNF-α, and IL-6, while increasing IL-10 levels, indicating that VITD reduced chondrocyte destruction and overcame harsh conditions in a dose-dependent manner.
Conclusion: Overall, the in vivo and in vitro findings show that VITD effectively reduces OA pain, inflammation, and chondrocyte destruction by lowering MMPs levels specifically.
Keywords: Chondrocytes; Inflammation; Knee osteoarthritis; Knee pain; Matrix metalloproteases; Vitamin D.
© 2023 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

