Polymeric copper(ii) and dimeric oxovanadium(v) complexes of amide-imine conjugate: bilirubin recognition and green catalysis
- PMID: 37124003
- PMCID: PMC10141293
- DOI: 10.1039/d3ra00702b
Polymeric copper(ii) and dimeric oxovanadium(v) complexes of amide-imine conjugate: bilirubin recognition and green catalysis
Abstract
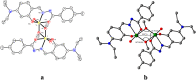
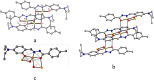
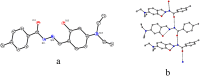
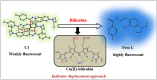
An exceptionally simple amide-imine conjugate, (E)-N'-(4-(diethylamino)-2-hydroxybenzylidene)-4-methylbenzohydrazide (L), derived by the condensation of 4-methyl-benzoic acid hydrazide (PTA) with 4-(diethylamino)-2-hydroxybenzaldehyde was utilized to prepare a dimeric oxo-vanadium (V1) and a one-dimensional (1D) copper(ii) coordination polymer (C1). The structures of L, V1 and C1 were confirmed by single crystal X-ray diffraction analysis. The experimental results indicate that V1 is a promising green catalyst for the oxidation of sulfide, whereas C1 has potential for a C-S cross-coupling reaction in a greener way. Most importantly, C1 is an efficient 'turn-on' fluorescence sensor for bilirubin that functions via a ligand displacement approach. The displacement equilibrium constant is 7.78 × 105 M-1. The detection limit for bilirubin is 1.15 nM in aqueous chloroform (chloroform/water, 1/4, v/v, PBS buffer, and pH 8.0).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no disagreement to mention.
Figures


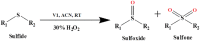


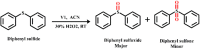

Similar articles
-
Exploring Anticancer and (Bio)catalytic Activities of New Oxovanadium(V), Dioxomolybdenum(VI), and Copper(II) Complexes of Amide-Imine Conjugates.ACS Appl Bio Mater. 2019 Jul 15;2(7):2802-2811. doi: 10.1021/acsabm.9b00226. Epub 2019 Jun 20. ACS Appl Bio Mater. 2019. PMID: 35030814
-
Oxovanadium(V) and Dioxomolybdenum(VI) Complexes of Amide-Imine Conjugates: Structures, Catalytic and Antitumor Activities.ACS Appl Bio Mater. 2019 Sep 16;2(9):3964-3973. doi: 10.1021/acsabm.9b00527. Epub 2019 Aug 27. ACS Appl Bio Mater. 2019. PMID: 35021329
-
Mo(vi) complexes of amide-imine conjugates for tuning the selectivity of fluorescence recognition of Y(iii) vs. Pb(ii).RSC Adv. 2022 Nov 21;12(51):33293-33303. doi: 10.1039/d2ra06035c. eCollection 2022 Nov 15. RSC Adv. 2022. PMID: 36425161 Free PMC article.
-
Synthesis, characterisation, reactivity and in vitro antiamoebic activity of hydrazone based oxovanadium(IV), oxovanadium(V) and mu-bis(oxo)bis{oxovanadium(V)} complexes.Dalton Trans. 2006 Feb 21;(7):937-47. doi: 10.1039/b512326g. Epub 2005 Nov 7. Dalton Trans. 2006. PMID: 16462954
-
Vanadium Complex Derived from N'-(3-Bromo-5-chloro-2-hydroxybenzylidene) -3-methylbenzohydrazide: Synthesis, Crystal Structure and Biological Activity.Acta Chim Slov. 2016;63(1):200-3. doi: 10.17344/acsi.2016.2266. Acta Chim Slov. 2016. PMID: 26970806
References
-
- Arthur G., The Amide Linkage: Selected Structural Aspects in Chemistry, Biochemistry, and Materials Science, Wiley-Interscience, 2000
-
- Humphrey J. M. Chamberlin A. R. Chem. Rev. 1997;97:2243–2266. - PubMed
-
- Liu X. Lin L. Feng X. Chem. Commun. 2009:6145–6158. - PubMed
-
- Pattabiraman V. R. Bode J. W. Nature. 2011;480:471–479. - PubMed
-
- Larock R. C., Comprehensive Organic Transformation, VCH, New York, 1999, 102
LinkOut - more resources
Full Text Sources
Research Materials

