A sigh-performance hydrogen gas sensor based on Ag/Pd nanoparticle-functionalized ZnO nanoplates
- PMID: 37124013
- PMCID: PMC10132452
- DOI: 10.1039/d3ra01436c
A sigh-performance hydrogen gas sensor based on Ag/Pd nanoparticle-functionalized ZnO nanoplates
Abstract
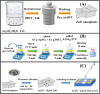
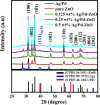
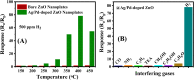
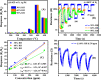
As a source of clean energy, hydrogen (H2) is a promising alternative to fossil fuels in reducing the carbon footprint. However, due to the highly explosive nature of H2, developing a high-performance sensor for real-time detection of H2 gas at low concentration is essential. Here, we demonstrated the H2 gas sensing performance of Ag/Pd nanoparticle-functionalized ZnO nanoplates. Bimetallic Ag/Pd nanoparticles with an average size of 8 nm were prepared and decorated on the surface of ZnO nanoplates to enhance the H2 gas sensing performance. Compared with pristine ZnO, the sensor based on ZnO nanoplate doped with Ag/Pd (0.025 wt%) exhibited an outstanding response upon exposure to H2 gas (R a/R g = 78 for 500 ppm) with fast response time and speedy recovery. The sensor also showed excellent selectivity for the detection of H2 over the interfering gases (i.e., CO, NH3, H2S, and VOCs). The superior gas sensing of the sensor was dominated by the morphological structure of ZnO, and the synergistic effect of strong adsorption and the optimum catalytic characteristics of the bimetallic Ag/Pd enhances the hydrogen response of the sensors. Thus, bimetallic Ag/Pd-doped ZnO is a promising sensing material for the quantitative determination of H2 concentration towards industrial applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







References
-
- Ahmad Fauzi A. S. Hamidah N. L. Sato S. Shintani M. Putri G. K. Kitamura S. Hatakeyama K. Quitain A. T. Kida T. Sens. Actuators, B. 2020;323:128678.
-
- Soboń A. Słyś D. Ruszel M. Wiącek A. Energies. 2021;14:7089.
-
- Cai L. Zhu S. Wu G. Jiao F. Li W. Wang X. An Y. Hu Y. Sun J. Dong X. Wang J. Lu Q. Jing Q. Liu B. Int. J. Hydrogen Energy. 2020;45:31327–31340.
-
- Zhou S. Ji J. Qiu T. Wang L. Ni W. Li S. Yan W. Ling M. Liang C. Inorg. Chem. Front. 2022;9:599–606.
-
- Arora K. Srivastava S. Solanki P. R. Puri N. K. IEEE Sens. J. 2019;19:8262–8271.
LinkOut - more resources
Full Text Sources
Research Materials

