The toxicity associated with combining immune check point inhibitors with tyrosine kinase inhibitors in patients with non-small cell lung cancer
- PMID: 37124513
- PMCID: PMC10140561
- DOI: 10.3389/fonc.2023.1158417
The toxicity associated with combining immune check point inhibitors with tyrosine kinase inhibitors in patients with non-small cell lung cancer
Abstract
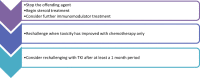
Latest advances in non-small cell lung cancer (NSCLC) therapies have revolutionized the treatment regimens utilized for NSCLCs with or without a driver mutation. Molecular targeted treatments such as tyrosine kinase inhibitors (TKIs) are utilized to prevent tumor progression and improve survival. Despite the great benefit of immunotherapy in NSCLC tumors with no driver mutation, the use of immune checkpoint inhibitors (ICIs) in NSCLC tumors harboring a driver mutation has been under debate. Furthermore, several trials have been conducted investigating the use of these therapies with TKIs. A few trials were halted due to growing concerns of increased toxicity with the combination of TKI and immunotherapy. The adverse events ranged from low grade dermatologic complaints to fatal interstitial lung diseases. These toxicities occur with both concurrent and sequential administration of treatment. Thus, recommendations for the safest method of combination treatment have not yet been described. This review paper discusses recent views on combination treatment, previous clinical trials reporting grade 3-4 toxicities, and guidelines for a safe timeline of administration of treatment based on past evidence.
Keywords: combination; immunotherapy; lung cancer; pneumonitis; toxicity; tyrosine kinase inhibitors.
Copyright © 2023 Kalra and Rashdan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Role of immunotherapy in metastatic EGFRm NSCLC: Is it relevant?Indian J Cancer. 2022 Mar;59(Supplement):S68-S79. doi: 10.4103/ijc.IJC_49_21. Indian J Cancer. 2022. PMID: 35343192 Review.
-
Toxicity Profile of Combining PD-1/PD-L1 Inhibitors and Thoracic Radiotherapy in Non-Small Cell Lung Cancer: A Systematic Review.Front Immunol. 2021 Mar 30;12:627197. doi: 10.3389/fimmu.2021.627197. eCollection 2021. Front Immunol. 2021. PMID: 33859637 Free PMC article.
-
Efficacy of immune checkpoint inhibitors in EGFR-Mutant NSCLC patients with EGFR-TKI resistance: A systematic review and meta-analysis.Front Pharmacol. 2022 Aug 22;13:926890. doi: 10.3389/fphar.2022.926890. eCollection 2022. Front Pharmacol. 2022. PMID: 36071838 Free PMC article.
-
The efficacy of immune checkpoint inhibitors in advanced non-small cell lung cancer harboring driver mutations.Mol Clin Oncol. 2019 Jun;10(6):610-614. doi: 10.3892/mco.2019.1838. Epub 2019 Apr 3. Mol Clin Oncol. 2019. PMID: 31031976 Free PMC article.
-
Immunotherapy Alone or in Combination with Chemotherapy as First-Line Treatment of Non-Small Cell Lung Cancer.Curr Treat Options Oncol. 2020 Jul 27;21(8):69. doi: 10.1007/s11864-020-00768-2. Curr Treat Options Oncol. 2020. PMID: 32720019 Review.
Cited by
-
Long-term safety of selpercatinib for Rearranged during transfection (RET)-activated advanced solid tumors in LIBRETTO-001: differing patterns of adverse events over time.Oncologist. 2024 Dec 6;29(12):1068-1078. doi: 10.1093/oncolo/oyae282. Oncologist. 2024. PMID: 39471424 Free PMC article. Clinical Trial.
-
Anti-EGFR aptamer exhibits direct anti-cancer effects in NSCLC cells harboring EGFR L858R mutations.NPJ Precis Oncol. 2024 Nov 21;8(1):271. doi: 10.1038/s41698-024-00758-9. NPJ Precis Oncol. 2024. PMID: 39572699 Free PMC article.
-
Osimertinib-Induced Hepatitis Following Immunotherapy in a Patient with Lung Adenocarcinoma Harboring De Novo EGFR Exon 19 Deletion and T790M Mutations: A Case Report.Reports (MDPI). 2025 Jun 26;8(3):101. doi: 10.3390/reports8030101. Reports (MDPI). 2025. PMID: 40700234 Free PMC article.
-
Efficacy of first-line immune checkpoint inhibitors in pulmonary sarcomatoid carcinoma.Transl Lung Cancer Res. 2024 Sep 30;13(9):2212-2221. doi: 10.21037/tlcr-24-263. Epub 2024 Sep 12. Transl Lung Cancer Res. 2024. PMID: 39430330 Free PMC article.
-
Case report: Single gene testing and comprehensive genomic profiling in non-small cell lung cancer-a case series of divergent results from a large reference laboratory.Front Oncol. 2024 Oct 31;14:1445668. doi: 10.3389/fonc.2024.1445668. eCollection 2024. Front Oncol. 2024. PMID: 39544293 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous