Oral fenofibrate for hyperbilirubinemia in term neonates: A single-blind randomized controlled trial
- PMID: 37125058
- PMCID: PMC10130838
- DOI: 10.1017/cts.2023.35
Oral fenofibrate for hyperbilirubinemia in term neonates: A single-blind randomized controlled trial
Abstract
Background: Hyperbilirubinemia is common in the neonatal period; however, delayed diagnosis or inadequate treatment can cause irreparable damage to the neonates. We aimed to evaluate the efficacy of oral fenofibrate for hyperbilirubinemia in term neonates.
Methods: This single-blind randomized controlled trial included 86 term neonates aged 3-7 days, with birth weight ≥2500 g, admitted to Bandar Abbas Children's Hospital, Bandar Abbas Iran, from July 23, 2019, to July 22, 2020. The fenofibrate group received 10 mg/kg oral fenofibrate and phototherapy, while controls only received phototherapy. Serum total bilirubin was measured at 24 and 48 h and at the time of discharge. Hospital length of stay was also noted.
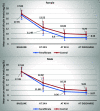
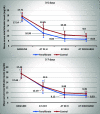
Results: The two study groups were comparable regarding age, gender, gestational age, birth weight, and baseline total serum bilirubin levels. Serum total bilirubin levels at 48 h (P < 0.001) and at discharge (P < 0.001) were significantly lower in the fenofibrate group compared to controls. Although hospital length of stay was lower in the fenofibrate group compared to controls, the difference was not statistically significant (P = 0.612). Fenofibrate was more effective on the reduction of serum bilirubin in neonates aged 3-4.5 days starting at the 24th hour. Moreover, it was more effective in female neonates compared to males starting at the 48th hour.
Conclusions: A single dose of oral fenofibrate reduced total serum bilirubin in term neonates with hyperbilirubinemia without any side effects; however, this effect was more prominent after 48 h.
Keywords: Fenofibrate; bilirubin; neonatal jaundice.
© The Author(s) 2023.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Geme JWS. Nelson Textbook of Pediatrics. Amsterdam: Elsevier. 2020.
-
- Olusanya BO, Teeple S, Kassebaum NJ. The contribution of neonatal jaundice to global child mortality: findings from the GBD 2016 study. Pediatrics 2018; 141(2): e20171471. - PubMed
-
- Sgro M, Campbell DM, Kandasamy S, Shah V. Incidence of chronic bilirubin encephalopathy in Canada, 2007–2008. Pediatrics 2012; 130(4): e886–e90. - PubMed
-
- Mreihil K, Benth JŠ, Stensvold HJ, Nakstad B, et al. Phototherapy is commonly used for neonatal jaundice but greater control is needed to avoid toxicity in the most vulnerable infants. Acta Paediatrica 2018; 107(4): 611–619. - PubMed
LinkOut - more resources
Full Text Sources
