A recombinant spike-XBB.1.5 protein vaccine induces broad-spectrum immune responses against XBB.1.5-included Omicron variants of SARS-CoV-2
- PMID: 37125241
- PMCID: PMC10133731
- DOI: 10.1002/mco2.263
A recombinant spike-XBB.1.5 protein vaccine induces broad-spectrum immune responses against XBB.1.5-included Omicron variants of SARS-CoV-2
Abstract
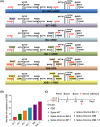
The XBB.1.5 subvariant has drawn great attention owing to its exceptionality in immune evasion and transmissibility. Therefore, it is essential to develop a universally protective coronavirus disease 2019 vaccine against various strains of Omicron, especially XBB.1.5. In this study, we evaluated and compared the immune responses induced by six different spike protein vaccines targeting the ancestral or various Omicron strains of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in mice. We found that spike-wild-type immunization induced high titers of neutralizing antibodies (NAbs) against ancestral SARS-CoV-2. However, its activity in neutralizing Omicron subvariants decreased sharply as the number of mutations in receptor-binding domain (RBD) of these viruses increased. Spike-BA.5, spike-BF.7, and spike-BQ.1.1 vaccines induced strong NAbs against BA.5, BF.7, BQ.1, and BQ.1.1 viruses but were poor in protecting against XBB and XBB.1.5, which have more RBD mutations. In sharp contrast, spike-XBB.1.5 vaccination can activate strong and broadly protective immune responses against XBB.1.5 and other common subvariants of Omicron. By performing correlation analysis, we found that the NAbs titers were negatively correlated with the number of RBD mutations in the Omicron subvariants. Vaccines with more RBD mutations can effectively overcome the immune resistance caused by the accumulation of RBD mutations, making spike-XBB.1.5 the most promising vaccine candidate against universal Omicron variants.
Keywords: Omicron; RBD mutations; SARS‐CoV‐2; XBB.1.5; neutralizing antibody; recombinant protein vaccine.
© 2023 The Authors. MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
This work was supported by the WestVac Biopharma Co. Ltd. Jiong Li, Wei Wang, Li Yang, Guobo Shen and Xiawei Wei are employees of WestVac Biopharma Co. Ltd. Remaining authors declare no conflicts of interest.
Figures





References
-
- Callaway E. Coronavirus variant XBB.1.5 rises in the United States—is it a global threat? Nature. 2023;613(7943):222‐223. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
