The Mammalian Circadian Time-Keeping System
- PMID: 37125558
- PMCID: PMC7614869
- DOI: 10.3233/JHD-230571
The Mammalian Circadian Time-Keeping System
Abstract
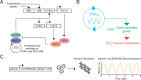
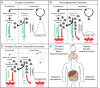
Our physiology and behavior follow precise daily programs that adapt us to the alternating opportunities and challenges of day and night. Under experimental isolation, these rhythms persist with a period of approximately one day (circadian), demonstrating their control by an internal autonomous clock. Circadian time is created at the cellular level by a transcriptional/translational feedback loop (TTFL) in which the protein products of the Period and Cryptochrome genes inhibit their own transcription. Because the accumulation of protein is slow and delayed, the system oscillates spontaneously with a period of ∼24 hours. This cell-autonomous TTFL controls cycles of gene expression in all major tissues and these cycles underpin our daily metabolic programs. In turn, our innumerable cellular clocks are coordinated by a central pacemaker, the suprachiasmatic nucleus (SCN) of the hypothalamus. When isolated in slice culture, the SCN TTFL and its dependent cycles of neural activity persist indefinitely, operating as "a clock in a dish". In vivo, SCN time is synchronized to solar time by direct innervation from specialized retinal photoreceptors. In turn, the precise circadian cycle of action potential firing signals SCN-generated time to hypothalamic and brain stem targets, which co-ordinate downstream autonomic, endocrine, and behavioral (feeding) cues to synchronize and sustain the distributed cellular clock network. Circadian time therefore pervades every level of biological organization, from molecules to society. Understanding its mechanisms offers important opportunities to mitigate the consequences of circadian disruption, so prevalent in modern societies, that arise from shiftwork, aging, and neurodegenerative diseases, not least Huntington's disease.
Keywords: Astrocyte; cryptochrome; feedback loop; hypothalamus; melanopsin; neurodegeneration; neuropeptide; period; retina; suprachiasmatic nucleus.
Conflict of interest statement
The authors have no conflict of interest to report.
Figures



References
-
- Czeisler CA, Gooley JJ. Sleep and circadian rhythms in humans. Cold Spring Harb Symp Quant Biol. 2007;72:579–97. - PubMed
-
- Hastings M, O’Neill JS, Maywood ES. Circadian clocks: Regulators of endocrine and metabolic rhythms. J Endocrinol. 2007;195(2):187–98. - PubMed
-
- Meyer N, Harvey AG, Lockley SW, Dijk DJ. Circadian rhythms and disorders of the timing of sleep. Lancet. 2022;400(10357):1061–78. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

